Wnt Signals are an Organizing Issue
* This paper was written upon reading a primary article published in the field of the author senior thesis project.
Embryonic development is a complex process. It now appears that signals generated at rhombomere boundaries regulate patterning of the zebrafish hindbrain.
During development, multicellular organisms generate an intricate and accurately patterned set of tissues from much simpler precursors. In the vertebrate hindbrain, similar cell types are repeated in succeeding compartments of hindbrain called rhombomeres1, but each compartment acquires its own identity and develops into a specialized region. The mechanisms that accomplish this feat are diverse, but in many cases still unknown. In the October (2004) issue of Developmental Dynamics, Riley et al. reveal that rhombomere boundaries serve as signaling centers that help regulate the reiterated pattern of similar cell types within the hindbrain2. Furthermore, the authors demonstrate that expression of wnt genes at rhombomere boundaries help regulate hindbrain patterning in the zebrafish Danio rerio, and delta signals from flanking cells provide feedback to maintain these boundaries2.
In the realm of development, boundary formation is an important regulator1. It coordinates patterns of differentiation in neighboring developmental compartments, and is often regulated by precise signaling fronts1. The best understood example of boundary formation occurs in the fruitfly Drosophila melanogaster3. In this model, the cells that will line the body surfaces are divided into a few spatially distinct compartments3. Specialized cells are then generated at the boundaries between compartments and act as organizers of further development, producing long-range signals that subdivide adjacent tissues into new regions and new signaling centers3.
Riley and collaborators revealed a similar mechanism in zebrafish by examining the role of wnt signals in hindbrain patterning. Wnt signals comprise a family of cysteine-rich glycoproteins constituting of fifteen genes in vertebrates4. Riley et al. determine that at least four of these wnt genes (wnt1, wnt3a, wnt8b, wnt10b) are up-regulated in cells at rhombomere boundaries during early hindbrain development2. Disruption of these wnt signals directly results in a disorganized metameric pattern2. Thus, coexpression of all four wnt genes is required for normal patterning.
To address boundary-specific functions, Riley et al. knocked out tcf3b (one of the wnt signaling mediators in the head)2. Following knockout, rhombomere boundaries are specifically disrupted and wnt up-regulation in boundary regions does not occur2. Consequently, tcf3b-deficient embryos are characterized by irregular patterning of cell types associated with rhombomeres, and a loss of rhombomere boundaries2.
Segmentation mutants spiel-ohne-grenzen (spg) and valentine (val) have been identified that resemble the defects evident in wnt-deficient and tcf3b mutants2. In these mutants, wnt genes are not up-regulated in segmental stripes2 and they do not form boundaries due to faulty interactions between neighboring rhombomeres2. Therefore, high levels of wnt are required at rhombomere boundaries in order to regulate patterning within each segment.
Riley et al. also examined the role of delta-notch signals (implicated as boundary formation regulators) in hindbrain patterning. Several delta genes (dlA, dlB, dlD) were identified in transverse stripes flanking rhombomere boundaries2. Generally, delta cells are inducing proteins that activate neighboring cells containing a notch protein in their cell membranes4. This notch protein then extends through the cell membrane to render a signaling cascade4. Delta-notch signals serve multiple functions in the hindbrain, but its best known function is in regulating neurogenesis2.Fronts of delta gene expression are usually associated with boundary formation in that they provide necessary feedback to stabilize long-range organizing signaling centers.
Riley et al. specifically evaluated delta signals along with wnt signals to elucidate the role of rhombomere boundaries in hindbrain development2. A mutant delta gene was created showing defects consistent with loss of rhombomere boundaries2. Similar defects are seen in mind bomb (mib) mutants, even though the mib phenotype is more severe2. These mib defects are consistent with the effects of disrupting rhombomere boundaries, and no apparent intermixing between adjacent segments occurs2. Thus, delta-signals seem to provide feedback to maintain wnt expression at rhombomere boundaries, and are thus important for metameric patterning of the hindbrain.
In order to determine if delta-notch signals maintain wnt signals, and consequently rhombomere boundaries, wnt signals would necessarily have to be expressed continuously in mib mutants to regain delta-notch signaling function2. Early mib mutants, injected with a plasmid containing wnt signals, showed substantial signs of rescue2. As a result, delta signals were maintained at high levels in these embryos and expressed more normal hindbrain morphology2. Thus, forced expression of wnt signals is responsible for rescue of mib mutants, and rescued mutants maintained better overall organization in the hindbrain2. However, once rhombomere boundaries are completely lost and patterning has become chaotic in late mib mutants, forced expression of wnt signals is not sufficient to regain normal patterning and function2. This indicates that wnt expression must be maintained for normal hindbrain development2.
In mib mutants, plasmid expression appears to have an effect on wnt expression by rescuing transverse stripes of wnt signals that appear to correspond with rhombomere boundaries but not rhombomere centers2. Therefore, a possible mechanism for wnt regulation of hindbrain patterning can be proposed. It is reasonable that wnt signal regulation at rhombomere boundaries involves an auto-regulatory feedback loop that helps maintain wnt expression (Figure 1). This auto-regulatory feedback loop is provided by delta signals from flanking cells that are required to maintain, sharpen, and refine patterning at rhombomere boundaries. Consequently, rhombomere boundaries act as signaling centers required for precise patterning of the hindbrain during development, and delta signals from flanking cells provide a feedback loop to maintain wnt expression at boundaries2.
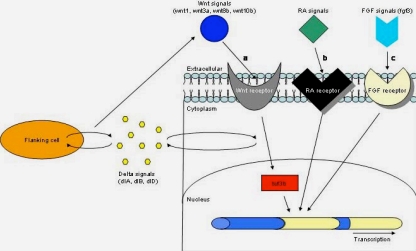
Figure 1. Mechanisms of hindbrain development
The pathway of zebrafish hindbrain development uncovered by Riley et al. is comparable to the previously identified mechanisms of hindbrain patterning in mouse and chick. (a) In zebrafish hindbrain, Wnt (wnt1, wnt3a, wnt8b, wnt10b) signals from rhombomere boundaries bind to their receptor, which acitivates tcf3b (a mediator of Wnt signaling in the nucleus), which becomes an active transcription factor that regulates metameric hindbrain patterning. Delta signals (dlA, dlB, dlD) from flanking cells provide feedback to maintain Wnt expression at boundaries. The less established models of mouse and chick hindbrain development can be summarized as follows: (b) in mouse and (c) chick, RA or FGF (fgf3) signals at rhombomere boundaries activate a signal transduction pathway that regulates hindbrain patterning through transcription.
In summary, Riley et al. examine the role of rhombomere boundaries as signaling centers that help organize hindbrain patterning. Four wnt genes are presumed to contribute to this patterning at rhombomere stripes during early embryonic development, and these stripes are maintained after the boundaries disappear2. Disruption of these genes ablates metameric patterning2. Furthermore, disruption of tcf3b, a signal mediator of wnt, also disrupts rhombomere boundaries similarly to wnt-deficient embryos2. This loss of function model reflects previously identified spg and val mutants, whereas delta-notch signaling morphants express a different phenotype2. In spg and val mutants, embryo dysfunction is attributed to faulty interactions between adjacent rhombomere segments2. In delta-notch signaling mutants, progressive deterioration of boundaries and chaotic patterning in the hindbrain is similar to the previously identified mib mutant2. Forced expression of wnt signals in mib mutants partially rescues hindbrain patterning as revealed by parallel stripes of delta gene expression, maintenance of wnt signals, and improvement of hindbrain morphology2. Together, these data indicate that boundary specific stripes of wnt signals are maintained by delta signals from flanking cells2.
The significance of the study performed by Riley et al. is invaluable. If rhombomere boundaries are not formed or maintained properly, various cell types including cranial nerves, ganglia, radial glia, commissural neurons, and reticulospinal neurons are mispatterned. In addition, the central nervous system would not function properly.
Traditionally, the role of rhombomere boundaries has been elusive. The data presented here suggests that a series of wnt signals induce or organize repeated patterns of similar cell types in successive rhombomere segments2. These data are supported by similar patterning events identified in the development of Drosophila body segments5 and the vertebrate limb bud6, which induces the apical ectodermal ridge. In Drosophila, the signals that produce boundary cells are required to prevent cells in different compartments from intermixing5. In vertebrates, rhombomere boundaries are induced by high levels of notch signaling and activating notch in non-boundary rhombomere cells causes these cells to migrate into the boundary region6. Furthermore, the similarities between these diverse model systems reflect a conserved development mechanism – a self-reinforcing signaling feedback loop that spatially organizes fields of cells3. It is still unclear whether wnt ligands play a role as organizing signals in all vertebrates since other signaling molecules, including RA7 and fgf38 in the mouse and chick, may also contribute to the organizing activities of rhombomere boundaries.
As is usually the case with pioneering work, the data from Riley et al. take the field of development biology considerably closer into the realm of hindbrain patterning. This research elucidates, at the very least, that local boundary signals are required for the development of commissural neurons and the organization of radial glia, the building blocks of important guidance cues for axonal pathfinding2.
Acknowlegements
I would like to thank Dr. Shubhik DebBurman and Dr. Diana Darnell for all of their wonderful insight, patience, and mentorship.
References
- Wingate, J.T., Lumsden, A. (1996). Persistence of rhombomeric organization in the postsegmentation hindbrain.Development122: 2143-2152.
- Riley, B.B., Chiang, M.Y., Storch, E.M., Heck, R., Buckles, G.R., Lekven, A.C. (2004). Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain.Dev. Dyn..231: 278-291.
- Irvine, K.D., Rauskolb, C. (2001). Boundaries in development: formation and function.Annu. Rev. Cell Dev. Biol. 17: 189-214.
- Gilbert, S.F. (2000). Developmental Biology, Sixth Edition. Sinauer Associates, Inc.,Sunderland,Massachusetts.
- Sanson, B. (2001). Generating patterns from fields of cells: examples from Drosophila segmentation.EMBO Rep.2: 1083-1088.
- Laufer, E., Dahn, R., Orozco, O.E., Yeo, C.Y., Pisenti, J., Denrique, D., Abbott, U.K., Fallon, J.F., Tabin, C. (1997). Expression of radical fringe in the limb bud ectoderm regulates apical ectoderm ridge formation.Nature386: 366-373.
- Holder, N., Hill, J. (1991). Retinoic acid modulates development of the midbrain-hindbrain border and affects ganglion formation in zebrafish embryos.Development113: 1159-1170.
- Mahmood, R., Kiefer, P., Guthrie, S., Dickson, C., Mason,I.(1995). Multiple roles for FGF-3 during cranial neural development in the chicken. Development 121: 1399-1410.
Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College.