Unveiling Correlation Between Behavioral Impairment and Neurodegeneration in Early Onset Alzheimer’s Disease
Background
Alzheimer’s disease (AD) is a human neurodegenerative disease linked to Aβ plaque formation and hyperphosphorylation of tau protein in neurofibrillary tangles and is the leading cause of dementia with an increasing socioeconomic burden on the world population [1,2]. AD is characterized by atrophy of the brain that starts with loss of tissue, neuronal death and microglial inflammation in the hippocampus which is also correlated with the progressive loss of cognition [3]. Late-onset AD makes up 98% of the cases and in which the symptoms arise after age 65, whereas early-onset AD (EOAD) usually starts before age 65 and progresses more rapidly making only 2% of the cases [2]. EOAD is often seen in patients with AD history which explains why it is also known as familial AD or FAD. Mutation in autosomal dominant genes APP [4], PSEN1 [5] and PSEN2 [6] are linked to development of FAD characterized by aberrant cleavage and aggregation of the amyloid precursor protein which consequently results in Aβ plaque formation [7]. The most prominent research model for AD is 5xFAD mice that express the 5 common mutations within some of the mentioned genes: APP(695) related Swedish (K670N,M671L), Florida (I716V), and London (V7171), and the PSEN1 related M146L and L286V [2]. It is crucial to mention that tau tangles are not observed in this model, so Aβ plaque is under focus [2]. Wild type Aβ does not express toxicity or tendency to aggregate and although not much is known about its primary function it is thought to be one of defense mechanisms in protecting the body from infection, repairing BBB leaks and regulating synaptic function [8, 9]. In healthy controls Aβ40 isoform is found in significantly higher amounts than Aβ42 and is easily soluble and degraded by CSF and vascular systems. However, when forming oligomers with other molecules Aβ starts forming plaques and becoming increasingly difficult to degrade, gaining the insoluble characteristics [9]. Multiple lines of research have examined the relationship between plaque aggregation, cognition and age, but the findings are still inconclusive for making usage of 5xFAD mice in preclinical research questionable [2, 10, 11]. Plaque accumulation is expected to start at the age of 2 months, data on cognitive impairment varies across studies [12, 13]. Furthermore, it has been long known that due to Thy1 promoter, female mice tend to develop more severe accumulation of Aβ plaques, but not much is known about the underlying mechanisms or the consequences per se [14, 15]. Studying sex related differences in Aβ pathology (Aβ40 and Aβ42) can be potentially linked to differences in cognition and age [12].
My goal in this research is to identify the relationship between plaque accumulation, sex and cognition measured in learning at ages 3-6 months in mice. The research model in use is 5xFAD mice from C57BL/6J strain. Using the IBL task, a standardized decision-making task in mice which has shown consistency across different studies, and revealed cognitive differences between young and old mice [16], this research will characterize the cognitive capacity of AD mice [17]. The findings in this research will enable us to get a better idea about how the behavioral changes refect the pathology of Alzheimer’s in mice and take us a step closer to understanding underlying mechanisms. I hypothesize that age and sex contribute to Aβ plaque accumulation and subsequently the onset of behavioral deterioration in 5xFAD mice.
Specific aims:
- Identify the cognitive changes in AD mice
- Assess the Aβ pathology in AD mice
- Evaluate the correlation between cognitive impairments and Aβ pathology.
All results will be compared to wildtype control mice to set the baseline for performance and brain condition. The male results will also be compared to females to have the comparative analysis as pointed out earlier.
Methods:
For aim 1: Following the standard protocols, we will surgically implant headbars in mice and then train them in the IBL task for 30 days (RFU IACUC protocol B23-15). Water-restriction is used as a motivation for the mice to perform and learn the IBL task. IBL training consists of four steps.Rig play is held for four days where the mouse is left to explore the task apparatus and get used to head fixing for 5 minutes. Habituation lasts for three days where the mouse is set in the rig for 15, 30, 45 minutes consecutively and observes the movement of the stimulus on the screen with the wheel taped. The mouse receives a water reward whenever the stimulus moves to the center. This step aims at showing the mouse the link between the stimulus on the screen and water reward. Easy-stimulus training is the first stage of training when mice are introduced to the task of rotating the wheel aiming to move the depicted stimulus to the middle of the screen. It begins with a stimulus with two contrasts 50% and 100%. Full-stimulus training is the final step after the mouse reaches 80% accuracy in each performance. New stimuli with contrasts lower than 50% are gradually introduced where the mouse will start relying on non-stimulus information such as previously shown stimulus, choice, and outcome. Aim 2: Once the behavioral experiment is complete, methoxy-x04 will be injected intraperitoneally (IP) 24 hours prior to perfusion to label Aβ plaque in green fluorescence. Perfusion and histology will be performed in accordance with the protocol. The brain slices will be analyzed through fluorescence microscopy. Aim 3: The extent of behavioral deficits analyzed from data obtained in aim 1 will be correlated with the extent of Aβ plaque pathology across mice.
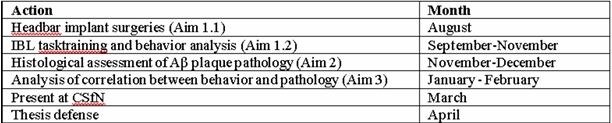
Table 1: Timeline for 2024-2025
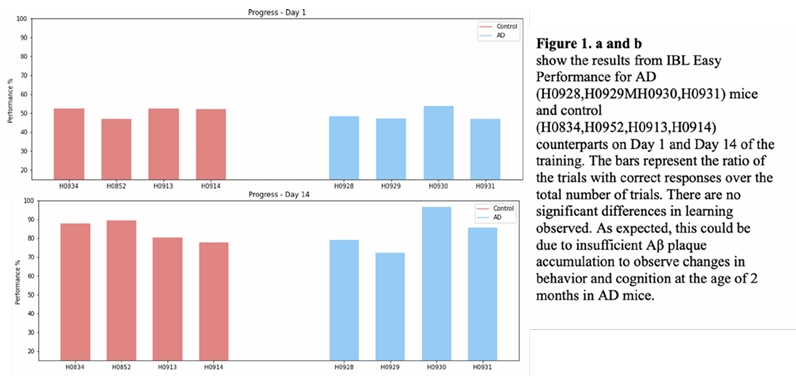
This spring, I started training 5xFAD and wild type mice on IBL task (Aim 2). The findings are not sufficient to make any conclusions.
Note: Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College. Articles published within Eukaryon should not be cited in bibliographies. Material contained herein should be treated as personal communication and should be cited as such only with the consent of the author.
References
Smith, S., & Hopp, S. C. (2023). The 5XFAD mouse model of Alzheimer’s disease displays age-dependent deficits in habituation to a novel environment. Aging Brain, 3, 100078.https://doi.org/10.1016/j.nbas.2023.100078
Oblak, A. L., Lin, P. B., Kotredes, K. P., Pandey, R. S., Garceau, D., Williams, H. M., Uyar, A., O’Rourke, R., O’Rourke, S., Ingraham, C., Bednarczyk, D., Belanger, M., Cope, Z. A., Little, G. J., Williams, S.-P. G., Ash, C., Bleckert, A., Ragan, T., Logsdon, B. A., & Mangravite, L. M. (2021). Comprehensive Evaluation of the 5XFAD Mouse Model for Preclinical Testing Applications: A MODEL-AD Study. Frontiers in Aging Neuroscience, 13. https://doi.org/10.3389/fnagi.2021.713726
Rao, Y. L., Ganaraja, B., Murlimanju, B. V., Joy, T., Krishnamurthy, A., & Agrawal, A. (2022). Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech, 12(2).https://doi.org/10.1007/s13205-022-03123-4
Walker, L. C. (2020). Aβ plaques. Free Neuropathology, 1, 31–31. https://doi.org/10.17879/freeneuropathology-2020-3025
Larner, A. J., & Doran, M. (2005). Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin–1 gene. Journal of Neurology, 253(2), 139–158. https://doi.org/10.1007/s00415-005-0019-5
Joshi, A., Ringman, J. M., Lee, A. S., Juarez, K. O., & Mendez, M. F. (2012). Comparison of clinical characteristics between familial and non-familial early onset Alzheimer’s disease.Journal of Neurology, 259(10),2182–2188. https://doi.org/10.1007/s00415-012-6481-y
Mendez, M. F. (2019). Early-onset Alzheimer’s disease and its variants. CONTINUUM: Lifelong Learning in Neurology, 25(1), 34–51. https://doi.org/10.1212/com
Rukmangadachar, L. A., & Bollu, P. C. (2019, February). Amyloid Beta Peptide. Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459119/
Brothers, H. M., Gosztyla, M. L., & Robinson, S. R. (2018). The Physiological Roles of Amyloid-β Peptide Hint at New Ways to Treat Alzheimer’s Disease. Frontiers in Aging Neuroscience, 10(118). https://doi.org/10.3389/fnagi.2018.00118
Sil, A., Erfani, A., Lamb, N., Copland, R., Riedel, G., & Platt, B. (2021). Sex differences in behavior and molecular pathology in the 5XFAD model. BioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2021.04.29.440396
Nehra, G., Promsan, S., Yubolphan, R., et al. (2024). Cognitive decline,Aβ pathology, and blood–brain barrier function in aged 5xFAD mice. Fluids Barriers CNS, 21(1), 29. https://doi.org/10.1186/s12987-024-00531-x
Chi Him Poon, Wong, N., Roy, J., Wang, Y., Wang, H., Steinbusch, H., Arjan Blokland, Yasin Temel, Luca Aquili, & Lee Wei Lim. (2023). Sex Differences between Neuronal Loss and the Early Onset of Amyloid Deposits and Behavioral Consequences in 5xFAD Transgenic Mouseas a Model for Alzheimer’s Disease. Cells, 12(5), 780–780. https://doi.org/10.3390/cells12050780
Forner, S., Kawauchi, S., Balderrama-Gutierrez, G., Kramár, E. A., Matheos, D. P., Phan, J., Javonillo, D. I., Tran, K. M., Hingco, E., da Cunha, C., Rezaie, N., Alcantara, J. A., Baglietto-Vargas, D., Jansen, C., Neumann, J., Wood, M. A., MacGregor, G. R., Mortazavi, A., Tenner, A. J., & LaFerla, F. M. (2021). Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Scientific Data, 8(1), 270. https://doi.org/10.1038/s41597-021-01054-y
Bhattacharya, S., Haertel, C., Maelicke, A., & Montag, D. (2014). Galantamine Slows Down Plaque Formation and Behavioral Decline in the 5XFAD Mouse Model of Alzheimer’s Disease. PLoS ONE, 9(2), e89454. https://doi.org/10.1371/journal.pone.0089454
Sadleir, K. R., Eimer, W. A., Cole, S. L., & Vassar, R. (2015). Aβ reduction in BACE1 heterozygous null 5XFAD mice is associated with transgenic APP level. Molecular Neurodegeneration, 10(1). https://doi.org/10.1186/1750-1326-10-1
Hwang, E. J., Korde, S., Han, Y., Sambangi, J., Lian, B., Owusu-Ofori, A., Diasamidze, M., Wong, L. M., Pickering, N., & Begin, S. (2023). Parietal stimulation reverses age-related decline in exploration, learning, and decision-making. BioRxiv: The Preprint Server for Biology, 2023.10.21.563408. https://doi.org/10.1101/2023.10.21.563408
The International Brain Laboratory, Aguillon-Rodriguez, V., Angelaki, D., Bayer, H., Bonacchi, N., Carandini, M., Cazettes, F., Chapuis, G., Churchland, A. K., Dan, Y., Dewitt, E., Faulkner, M., Forrest, H.,Haetzel, L., Häusser, M., Hofer, S. B., Hu, F., Khanal, A., Krasniak, C.,& Laranjeira, I. (2021). Standardized and reproducible measurement of decision-making in mice. ELife, 10, e63711. https://doi.org/10.7554/eLife.63711