Science of Teaching: X-Ray Fluorescence and Moseley’s Law
From ancient artifacts to modern Gadgets, X-ray Fluorescence (XRF) is a powerful scientific tool that allows us to peek inside materials and understand what they are made of. We can uncover their hidden secrets, guiding archaeological digs, geological surveys, and industrial processes alike. We will explore why XRF is so important, how it works, and its applications in fields like archaeology and geology, and consider the big questions it helps us answer about the world around us.
X-rays were discovered by German physicist Wilhelm Conrad Roentgen in 1895 while experimenting with cathode ray6 . X-rays are a form of electromagnetic radiation, similar to light but with much higher energy. They are invisible to the human eye and can pass through most objects, including the human body. XRF uses X-rays in a blend of physics, chemistry, and technology that lets scientists analyze the elements present in a wide range of substances. This allows scientists to examine the elemental composition of materials without damaging them. It is similar to having a microscope that can see atoms, the building blocks of matter.
Henry Moseley, born in 1887 in Weymouth, England, was a pioneering physicist whose groundbreaking work revolutionized our understanding of the atom5 . While studying at the University of Oxford, he conducted experiments under Ernest Rutherford, leading to the discovery of the relationship between X-ray spectra and atomic number, known as Moseley’s Law. This fundamental breakthrough laid the groundwork for the modern periodic table and significantly advanced the field of atomic physics. Tragically, Moseley’s promising career was cut short when he was killed in action during World War I at the age of 27, leaving behind a lasting legacy in science.
X-ray Fluorescence is a technique that works by shooting X-rays at a sample, which excites atoms inside (Figure 1). These excited atoms then emit characteristic X-rays, which can be detected and analyzed to determine what elements are present in the sample. Because of the way an atom’s electrons are arranged in shells around the nucleus when an electron from a higher energy shell drops down to fill a vacancy in a lower energy shell, it releases energy in the form of an X-ray photon. This energy corresponds to the difference in energy levels between the two shells involved in the transition.
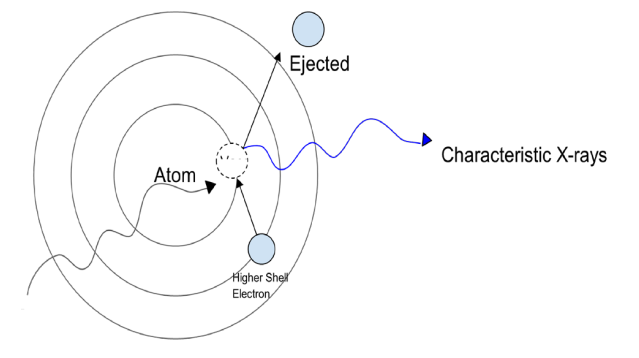
Figure 1. An incident X-Ray, causing ejection of ground state electron, higher shell electron fills in vacant spot releasing characteristic X-Rays.
The equipment used helps achieve our goals by emitting X-Rays into a select sample. That sample’s atoms emit their unique X-Rays (Figure 1) which can be interpreted by a detector. The computer then takes that interpretation and displays it on a graph that scientists can analyze (Figure 2 and Figure 3).
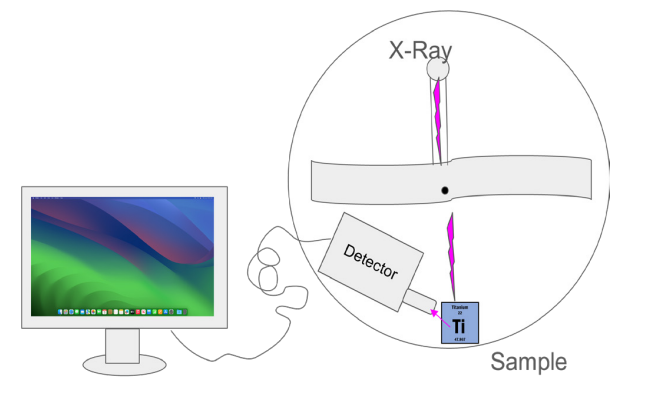
Figure 2. Apparatus with an X- Ray Source pointed at a Titanium sample.
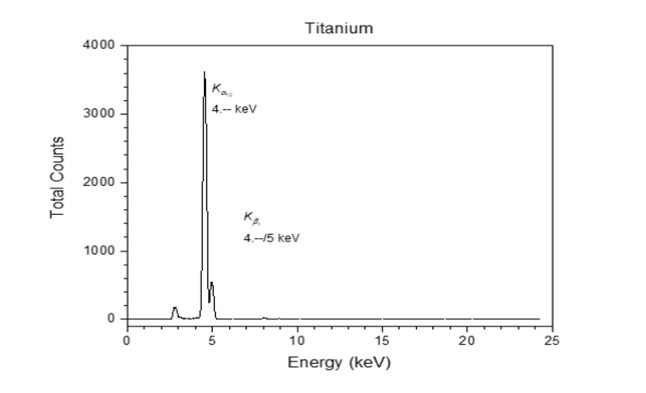
Figure 3. Example spectrum graph of a Titanium element sample.
XRF serves as a critical tool for evaluating fundamental scientific principles such as Moseley’s Law, which elucidates the correlation between the energy of characteristic X-rays and the atomic number of an element denoted by (Z - b)2 where ‘Z’ is the atomic number and ‘b’ is the shielding constant.[1,2,3] Scientists design and execute experiments employing XRF, directing X-rays at materials of known composition (Figure 2) and subsequently distilling the results against the theoretical predictions derived from Moseley’s Law. This process contributes significantly to validating our comprehension of atomic structure and the underlying principles of physics such as quantum mechanical functionality - particularly through the behavior of electrons, energy levels, and transitions - re-conceptualizing the periodic table.
Following the execution of experiments, and rigorous data analysis, scientists derive conclusions regarding the fidelity of Mosely’s Law, particularly in the significance of the shielding constant. The shielding constant lies in its influence on the energy levels and arrangement of electrons within atoms. It helps explain phenomena such as the ordering of elements in the periodic table and the progression of electron configurations within elements. Through comparative analysis with established benchmarks, these findings yield profound insights into the intricacies of atomic structure, furthering our understanding of the inherent natural behavior.
The outcomes derived from these experiments bear substantial significance for future scientific investigation and practical applications of XRF. Pottery and ceramic artifacts are abundant in archaeological sites and provide valuable insights into past civilizations, cultures, and technological advancements. XRF helps date these ancient artifacts and identify the materials they’re made of, as well as their manufacturing techniques and geographical origins. In Geology, it aids in mineral analysis, ore exploration, and mapping the Earth’s surface, helping us understand our planet’s history and resources. One example that fulfills all these investigations is the analysis of the obsidian in its ore form and tools4 . XRF catalyzes the refinement of analytical techniques, the development of innovative technologies, and the resolution of real-world challenges spanning diverse domains.
In summation, X-ray Fluorescence emerges as a strong tool, facilitating the exploration of the concealed properties inherent in materials. By fully understanding the operational mechanisms of XRF, and leveraging its capabilities to validate scientific principles, we are positioned to unravel the mysteries of the universe and proceed in advancements that add to the benefit of society at large.
Note: Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College. Articles published within Eukaryon should not be cited in bibliographies. Material contained herein should be treated as personal communication and should be cited as such only with the consent of the author.
References
1 Tipler and Llewellyn, Modern Physics, 4 e (Freeman, New York, 2003) pp. 186-190
2 S.B. Gudennavar, N.M. Badiger, S.R. Thontadarya and B. Hanumaiah, Am. J. Phys. 71, 822 (2003).
3 M.A.B Whitaker, Eur. J. Phys 20, 213 (1999)
4 Sayre, Edward V., Timothy A. Kohler, Michael D. Glascock, Ronald L. Bishop, and Aaron R. Norby. “Sourcing ceramics with portable XRF spectrometers: A comparison with INAA using Mimbres pottery from the American Southwest.” Journal of Archaeological Science 35, no. 10 (2008): 2758-2772.
5 Encyclopedia Britannica. s.v. “Henry Gwyn Jeffreys Moseley,” accessed February 26, 2024,
6 Roentgen, Wilhelm Conrad. “On a New Kind of Rays: A Preliminary Communication.” In Scientific Papers of Wilhelm Conrad Roentgen, translated by Albert von Lichtenberg, 1-6. London: George Bell and Sons, 1899.