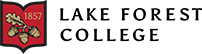Ontogeny of the Lateral Line and Skeletal System in Various Age Groups of Poecilia reticulata: Insights from Fluorescent Imaging, Diaphonization, and Behavioral Trials
*These authors wrote this paper for Neuroscience 340: Animal Physiology taught by Dr. Margot A. B. Schwalbe.
Introduction
The model organism used for this project is Poecilia reticulate chosen for its abundance, essential when considering the relatively high number of fish required to address the aims of this study. Poecilia reticulate commonly known as guppy, is a small teleost fish found in the freshwaters of the northeast region of South America. They are highly prolific, allowing for adaptation in various environmental conditions. Additionally, they are live breeders with a short life cycle. This feature makes them one of the ideal model organisms in aquatic vertebrate studies (Mizoguchi et al., 2022). Despite extensive research on guppies, no recorded literature has studied the ontogeny of the lateral line system and skeletal structure and connected it to functional behavior.
Guppies are born with developed jaws, fins, and eyes, as well as a lateral line system that can help them avoid predators, thus raising the question of how the lateral line system evolves with age (Mousavi-Sabet et al., 2014). Yet, there is no visual mapping of Poecilia reticulate’s neuromasts throughout development (Fuiman et al., 2004). This is an important reason for our group to study their developmental morphology, specifically lateral line system, bone structure, and behavioral escape response.
The neuromasts in the cranial canal increase in both size and volume throughout development (Chang et al., 2018; Webb & Shirey et al., 2003). Previous research found that Danio rerio, commonly known as zebrafish, showed increased bone thickness as they age from adolescence to adulthood (Chang, Chuang, & Akhtar et al., 2018). Researchers have also mapped the skeleton of other species of fish such as zebrafish using the clear and stain procedure to investigate sexual dimorphism in the fish skeleton. Yet little is known about the evolution of the skeleton of Poecilia reticulate as the fish matures (Bird and Mabee, 2003; Jalili and Eagderi, 2017).
Similarly, responses to various stimuli may differ with the age of the fish. For example, a study by (Scott et al., 2023) showed that variation in canal and superficial lateral line morphology in different aged fish impacted their escape response. Escape responses in fish are studied in various methods, one of which involves exposing them to an oscillating probe in the tank, to elicit an escape response called the C-escape response (Tytell & Lauder, 2008). From previous research, we know that the lateral line system is the most reactive to 20-50 Hz (Weeg & Bass, et al., 2002). In some studies, introducing such stimulus causes the fish to turn their body into a C-shape away from the stimulus (Tytell & Lauder, 2008). It is not known whether Poecilia reticulate exhibits a similar response. However, any response can be dependent on the degree of lateral line development.
Here, it is hypothesized that the lateral line system and the skeletal structure of Poecilia reticulata would get more complex with advancing life stages. Moreover, adult fish will exhibit a greater escape behavior than juveniles due to the increasing development and complexity in the types of neuromasts that make up the complete lateral line system.
If the lateral line is less developed in juvenile Poecilia reticulata, then there will be a lower number of neuromasts visible under fluorescent imaging in comparison to adult fish. Similarly, if Poecilia reticulata is an earlier life stage, its skeletal structure will be less dense than the adult which can be observed upon diaphanization or clearing and staining. Lastly, if the Poecilia reticulata has a lower number of neuromasts that make up the lateral line system, then they will exhibit a smaller escape response when reacting to an oscillating probe (50 or 60 Hz, 10 V). In contrast, they will spend more time away from the stimulus if they have a more developed lateral line system.
Materials and Method
We acquired Poecilia reticulate from a fish tank located in the Lillard Science Center at Lake Forest College. Because the fish were not tracked from birth, their age or life stage was estimated based on their body size. The same guppy individuals were used for fluorescent imaging and clear-and-staining, while different individuals for behavioral trials.
2.1 Visualizing the Lateral Line System
The lateral line system of Poecilia reticulate was visualized by staining different-sized fish (n=3) with a vital fluorescent dye 63 µM 4-4(-diethtylaminostyryl)-1-methylpyridinium iodide (4-di-2-asp; CAS Number 105802-46-8, MW = 394.29 g/mol) solution for 5 minutes. For high-quality imaging, the fish were euthanized with 0.02% Tricane in conditioned tap water (MS-222; CAS Number 886-86-2, MW = 261.29 g/mol). The fish were added to a sylgard-coated petri dish covered with diluted MS-222 solution and imaged under a fluorescence microscope using a GFP fluorescence filter. The digital images were captured using ACCU-SCOPE and the camera settings, i.e., exposure control, and image processing were controlled using Captavision+ software. Images were taken from three views, dorsal, lateral (left and right), and ventral, and auto-stitched using Adobe Photoshop CS. The magnification of all images stitched together was 9.10. The length of the juvenile guppy was 1.5 ± 1 cm, the adult male was 2.2 ± 1 cm and the adult female was 3.7 ± 1 cm. The fluorescent staining protocol was provided by the laboratory instructor Dr. Schwalbe in lab 5 (2024).
2.2 Quantifying the Lateral Line
The stitched images were divided into three different views: lateral, dorsal, and ventral. All images were scaled to 5 mm. The neuromasts were categorized either as canal or superficial. A labeled canal of Astatotilapia burtoni was used as a reference guide to label the canal neuromasts of the guppies (Butler et al., 2015) which consisted of suborbital (SO), infraorbital (IO), otic (OT), mandibular-pre-opercular (MD-PR) and trunk (T) and supratemporal (ST). Each of the six canal neuromasts was found in adult male and female guppies, but not in juveniles. Any neuromasts not present in the canals were referred to as superficial neuromasts. For data collection, we started by counting all the canal neuromasts in lateral (left and right) views, followed by dorsal and ventral views. We ensured not to re-count the same neuromasts when counting from a different view. The same procedure was conducted for counting the superficial neuromasts for all three fish. The total number of neuromasts in each fish was plotted in Figure 7. The proportion of canal vs superficial neuromasts from the total number of neuromasts in each fish was plotted in Figure 8.
2.3 Visualizing Skeletal Structure
First, four fishes of different sizes used in fluorescent imaging were obtained. Next, the clearing and staining protocol was followed to stain the fish skeleton. In this protocol, each fish is put into a sealed container containing alcian blue in acetic acid and EtOH for 24 h or less depending on fish size (< 80 mm is less). Next, they are neutralized by being placed in saturated sodium borate solution for between 12h to 24h, again, depending on their size. Followed by bleaching in 15 mL of 3% hydrogen peroxide (H2O2) in 85 mL of 1% potassium hydroxide (KOH) for 20-40 min if the fish is < 80 mm or 1-1.5 h if > 80 mm. Trypsin digestion is conducted by adding the fish in 1g of trypsin in 35 mL saturated sodium borate solution and 65 mL distilled water until fish vertebrae are slightly visible. Next, the bones were stained using alizarin red in 1% potassium hydroxide (KOH) for 1 day if the fish was smaller than 80mm, 2 days if between 80 and 200mm, and 4 days if larger than 200mm. The next step was distaining with 1g of trypsin until the excess stain was completely removed. Lastly, the fishes were transferred into 30 mL glycerin in 1% KOH (70 mL) for 1.5 days, then 60 mL glycerin in 1% KOH (40 mL) for 1 day, and finally in 100% glycerin with thymol for storage (80% glycerin in 1% KOH (20 mL) may also be used alternatively). Storage was performed at room temperature in airtight containers with mild agitation once a day (Schwalbe, 2024).
After staining, the fish were viewed under a light microscope at 0.6 magnification. The surface area of the fish skeleton and the total body area were measured from the obtained images using the free-form tool on ImageJ after calibrating the scale on the application. Each measurement was done 3 times, and the average value was used as the actual area recorded for data analysis.
The skeleton consisted of the spine area plus the skull area on the lateral view, which were measured separately because it made tracing with ImageJ more accurate and manageable. The ribs and the fins were excluded from the skeletal surface area calculation after unsuccessful attempts to trace them. To maintain consistency, the fins were also excluded from the total surface area (See Fig. 1). Lastly, the ossification bone area percentage was plotted on a bar graph in Excel. No statistical analysis was conducted because there was only one fish per size.
2.4 Behavioral Trials
We designed an experiment to measure a reaction to a stimulus to test whether the younger fish have fewer neuromasts through behavioral trials. The setup included an area with a black curtain, opaque bucket, lamp for a light source, oscillating probe, and video camera as illustrated in Fig. 2. We borrowed an oscillating probe from the physics department at Lake Forest College. The probe is a wooden stick with a wire and a white ball attached. Only the white ball and wire were submerged in water. The power unit can be set to different frequencies (Hz) and voltages (V). In total, we tested five fish.
We put an individual fish into a bucket with tank water and left it to accumulate in a new environment for 15 minutes. Then, we turned on the probe for 30 seconds at 60 Hz, 10 V. The fish was left to rest for 1 minute and then exposed for 30 seconds to the probe at 50 Hz, 10 V. Only data from 50 Hz was quantified. These frequencies were chosen based on research by Weeg and Bass (2002) which showed that the lateral line system is responsive to a range of 20-50 Hz. Afterward, we analyzed the videos.
The length of each fish was measured using the ImageJ program. The video analysis was conducted by dividing the bucket into quadrants (Q), with the ones farthest from the stimulus labeled Q1, Q2, Q3, and Q4. Q2 and Q3 were the farthest from the stimulus. The probe was placed in the middle of the line dividing Q1 and Q4 as shown in Fig. 3. For every second of the recording, the quadrant where the fish was located was recorded in Microsoft Excel and plotted. A statistical analysis was performed using the JMP software.
3. Results
3.1 Lateral line development
The fluorescent images of guppies revealed canal neuromasts in the anterior (head) and posterior (trunk) regions of the body. In juvenile guppy, we observed three types of canal neuromasts: suborbital and infraorbital in open bony canals in the head, along with a presumptive trunk canal in the body (Fig. 4). In adult male guppy, we observed six types of canal neuromasts: suborbital, infraorbital, supratemporal, mandibular-pre-opercular, and otic in head and two trunk canals running along the body (Fig. 5). Similarly, in adult female guppy, we observed all six types of canal neuromasts observed in adult male guppy (Fig. 6). We found that adult guppies develop 2 trunk canals.
After quantifying the number of neuromasts, we found that as the lateral line system develops in juveniles, the number of neuromasts increases. Juveniles had the lowest number of neuromasts compared to adult (male and female) fish (Fig. 7). Despite having a larger body size, adult female guppy (3.7 cm) had a lower number of total neuromasts compared to adult male guppy (2.2 cm). Adult male guppy had the highest number of neuromasts (Fig. 7).
We were further interested in looking at the distribution of these neuromasts in each of the fish’s body. Juvenile fish had canal neuromasts and lacked superficial neuromasts. The difference in the number of canal neuromasts between adult male and female guppies was small compared to the number of superficial neuromasts. Adult males had the highest number of both superficial and canal neuromasts compared to adult females. Lastly, the number of superficial neuromasts was greater than canal neuromasts in male fish. Whereas the number of canal neuromasts was greater than superficial neuromasts in female fish (Fig. 8).
3.2 Skeletal Development
The results show that fish have increased calcified bone (red) and decreased cartilage (blue) as they become larger (Fig. 10). The smallest fish studied only shows cartilage, which is maintained at the ends of the fins, at the area where the fins meet the body of the fish, and on the ventral aspect of all larger fishes (Fig. 10). Looking at the percentage of fish skeleton surface area, there is a major increase between the smallest 1.5cm (0%) and the second smallest, 1.7cm fish (32%) but no difference from the second smallest and other larger fishes (Fig. 11).
3.3 Behavioral Responses
We quantified the data as the number of fish spent in each of the four quadrants. In Fig. 12, we plotted the data as a bar graph showing the individual fish by size (cm) and time (sec). From qualitative analysis, there is no direct trend, but the larger fish at 2.1 and 2.2 cm tended to spend more time in Q2 away from the stimulus. First, at 1.7 and 1.6 cm, I spent most of the time in Q4 near the stimulus. We ran a two-way ANOVA run to analyze this data further.
A two-way ANOVA was performed to evaluate the effects of fish size and quadrant location on time spent in each quadrant. Each trail is an individual fish, and we did not replicate it. The results indicated no significant interaction between fish size and quadrant location, F(7) = 0.1313, p = 0.994 (Fig. 13). Due to the high p-value, we do not have evidence to reject the null hypothesis.
4. Discussion
Our experiment explores the intricate process of sensory development in fish, with a specific focus on the development of superficial and canal neuromasts, and its potential ecological implications during a critical life stage. By tracking this sensory development, we illuminate how fish adapt to their environments, particularly their ability to detect stimuli such as water flow, pressure changes, and prey movement. This discovery holds profound significance for both scientists and the public.
The data analysis from the quantification of neuromasts indicates that adult fish have a greater abundance of both canal and superficial neuromasts than juveniles. Like other teleost species, guppies undergo the development of their presumptive canal neuromasts first, followed by the development of superficial neuromasts (Webb and Shirey et al., 2003). From observation, not all cranial canal neuromasts develop synchronously. The findings from fluorescent imaging of juvenile guppy suggest that suborbital and infraorbital are formed to the closest extent to maturity while the trunk canal was on its earliest track of development.
Furthermore, there was a difference in the number of superficial neuromasts between adult males and females. Driven by both behavioral adaption and sexual selection, male guppies tend to be more colorful than females. Females prefer colorful male guppies for mating. According to one study examining the sexual selection theory, female guppies preferred mating with the brightest male guppies. The prediction was that brightly colored males acquire greater fitness which is favored by sexual selection (Godin et al., 1996). Greater fitness equates to an enhanced ability to evade predators and illicit escape response. Therefore, sexual selection pressure may have led male guppies to adopt a greater abundance of neuromasts.
The experiments carried out in this project revealed increased ossification of bone with aging. The juveniles showed more cartilage (blue) than bones (red) compared to young adults, and full adults had a greater bone in proportion to cartilage. Therefore, the intensity of bone ossification increases with aging. As the bony canal becomes fully developed, it can accommodate more canal neuromasts. Therefore, we see increased differentiation in cranial canal neuromasts and two different trunk canals in both adult guppies compared to juveniles. Our findings are consistent with the literature reporting increased bone density and thickness in fish as they age through calcification, or ossification, of skeletal bones (Chang et al., 2018, Sæle et al., 2017).
During preliminary trials, we found that the Poecilia reticulata mainly exhibited some response to the oscillating probe when set to 10 V at 50 Hz. For example, from quantitative observations, fish 3 (2.2 cm), when exposed to 50 Hz, would stay far away from the stimulus and swim back and forth around the edge of the bucket. On the other hand, fish 1 (2.1 cm) started jumping out on the edges of the bucket when the same stimulus was present. Other fish swam to the stimulus instead or did not care and swam around the bucket.
These observations illustrate individual differences between the fish. However, there are many confounding variables as to why we observed no trend. For example, in some trials, the fish is first exposed to 60 Hz, which may affect the initial response. The analysis was that the fish may have been in multiple quadrants during the second as it swims fast. We also had a limited sample size of only five fish, which will not account for individual variance. In the paper by Weeg and Bass (2002), they exposed the fish to different time intervals, so the 30 seconds may have been too long to observe the initial response. Lastly, not all fish started in the same quadrant—notably, the fish at 1.7 cm in Q4 and the other four fish started in Q2. Looking at Fig. 12, this was the only fish that spent 30 seconds next to the stimulus, indicating that the fish’s location when the probe turned on may matter.
Our experiment goes beyond mere anatomical observations to explore the functional aspect of sensory development. Through behavioral trials and fluorescence imaging, we investigate the correlation between sensory maturation and behavioral responses in guppies. This integrative approach not only deepens our understanding of sensory function but also offers a comprehensive framework for studying sensory development in other aquatic organisms.
5. Future Studies
In future studies, we plan to expand on our current findings to explore the developmental morphology of Poecilia reticulate further. We will focus on the functional aspects of sensory development, particularly neuromast development through behavioral trials. By conducting more extensive experiments under various stimuli, we aim to understand how sensory maturation influences guppies’ adaptive strategies in their natural environment.
We aim to expand on ecological implications to include factors like habitat preferences and predator-prey interactions that will provide broader insights into sensory development in aquatic ecosystems. We also aim to improve quantitative analyses by expanding on neuromast quantification and refining bone measurement methods, possibly incorporating fin bones. It would be interesting to track the lateral line development of fish from larval stage to sexual maturity, by isolating a group of larvae since birth and performing fluorescent imaging throughout their course of development. This would give us a greater understanding of the timeline required for the differentiation of cranial canal neuromasts.
Plan on investigating gender differentiation in juvenile guppies to understand potential gender-specific differences in neuromast development and how it may relate to behavioral or other evolutionary adaptations, i.e., sexual selection. We will further explore individual variations in behavioral responses by conducting more trials at different intervals and assessing additional frequencies to detect differences suggested by previous research. Weeg and Bass’s (2002) paper states that the lateral line detects 20-50 Hz, prompting us to assess more frequencies to determine if there are observable differences.
These studies aim to uncover the intricate mechanisms underlying sensory adaptations and ecological dynamics in guppy populations, contributing to our understanding of their ecological significance in aquatic environments.
6. Conclusion
Our study explored the ontogeny of the lateral line system and skeletal structure of Poecilia reticulate, revealing insights into their developmental morphology and its link to functional behavior. We conducted a comprehensive examination involving fluorescent imaging, neuromast quantification, skeletal visualization, and behavioral trials. Through these techniques, we uncovered intriguing patterns in sensory structure development and their ecological implications.
Our findings revealed a clear developmental trajectory in both the lateral line system and skeletal structure of guppies. With maturation, fish exhibited a noticeable increase in the complexity of their lateral line system, showing more neuromasts, especially in the cranial canal regions. Similarly, skeletal development shifted from predominantly cartilaginous to calcified bone as fish progressed from juveniles to adults. This increase in bone density may correspond to the accommodation of canal neuromasts, highlighting the interconnected nature of sensory and skeletal development in aquatic vertebrates. Our hypotheses regarding the skeletal structure gaining complexity with advancing life stages and the lower density of neuromasts in younger Poecilia reticulata compared to adult fish were both supported by our findings.
Meanwhile, cartilage maintenance at the end of fish fins suggests a functional reason. Softer cartilage may allow for better flexibility in these specific areas, which would increase fish movement flexibility as an organism, thus potentially providing a better survival rate when the fish is being hunted by predators (Mousavi-Sabet et al., 2014).
The behavioral trials provided fascinating insights into how sensory maturation influences guppies’ behavioral responses. Although no direct trend was observed, larger fish tended to spend more time away from the stimulus, hinting at a potential correlation between sensory development and escape behavior. These nuanced findings emphasize the importance of considering both anatomical and functional aspects of sensory development in understanding the adaptive strategies of aquatic organisms. The findings regarding the relationship between neuromast density and escape response were inconclusive, as behavioral trials showed varied responses. This indicates that other factors may influence escape behavior in Poecilia reticulata, and our initial hypothesis was not supported.
In essence, our research opens new avenues for exploring the intricate mechanisms underlying sensory development in aquatic organisms, paving the way for future investigations into the ecological significance of sensory adaptations in Poecilia reticulate populations.
Note: Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College. Articles published within Eukaryon should not be cited in bibliographies. Material contained herein should be treated as personal communication and should be cited as such only with the consent of the author.
References
Bird, N. C., & Mabee, P. M. (2003). Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Developmental Dynamics, 228(3), 337–357. https://doi.org/10.1002/dvdy.10387
Chang, Z., Chen, P. Y., Chuang, Y. J., & Akhtar, R. (2018). Zebrafish as a model to study bone maturation: Nanoscale structural and mechanical characterization of age-related changes in the zebrafish vertebral column. Journal of the Mechanical Behavior of Biomedical Materials, 84, 54–63. https://doi.org/10.1016/j.jmbbm.2018.05.004
Fuiman, L. A., Higgs, D. M., & Poling, K. (2004). Changing structure and function of the ear and lateral line system of fishes during development. American Fisheries Society Symposium, 2004, 40, 117-144. https://scholar.uwindsor.ca/biologypub/115
Godin, J. G., & Dugatkin, L. A. (1996). Female mating preference for bold males in the guppy, Poecilia reticulata. Proceedings of the National Academy of Sciences of the United States of America, 93(19), 10262–10267. https://doi.org/10.1073/pnas.93.19.10262
Henríquez Martínez, A., Ávila, L. C., Pulido, M. A., Ardila, Y. A., Akle, V., & Bloch, N. I. (2022). Age-Dependent Effects of Chronic Stress on Zebrafish Behavior and Regeneration. Frontiers in Physiology, 13, 856778. https://doi.org/10.3389/fphys.2022.856778
Jalili, P. and Eagderi, S. (2017) Study of the Sexual Dimorphism in the Skeleton of Guppy, Poecilia reticulata (Poeciliidae). Poeciliid Research, 7, 147-155.
http://www.pr.bioflux.com.ro/docs/2017.147-155.pdf
Mousavi-Sabet, H., Azimi, H., Eagderi, S., Bozorgi, S., & Mahallatipour, B. (2014). Growth and morphological development of guppy Poecilia reticulata (Cyprinodontiformes, Poeciliidae) larvae. International Journal of the Bioflux Society, 4(1).
Sæle, H., T., Karlsen, van der Meeren, T., Bæverfjord, G., Hamre, K., Rønnestad, I., Moren, M., & Lie, K. K. (2017). Ossification of Atlantic cod (Gadus morhua) – developmental stages revisited. Aquaculture, 468, 524–533. https://doi.org/10.1016/j.aquaculture.2016.11.004
Tytell, E. D., & Lauder, G. V. (2008). Hydrodynamics of the escape response in bluegill sunfish, Lepomis macrochirus. Journal of Experimental Biology, 211(21), 3359–3369. https://doi.org/10.1242/jeb.020917
Webb, J. (2011). HEARING AND LATERAL LINE | Lateral Line Structure. Encyclopedia of Fish Physiology, 1, 336–346. https://doi.org/10.1016/b978-0-12-374553-8.00010-1
Imai, M., Mizoguchi, T., Wang, M., Li, Y., Hasegawa, Y., Tonoki, A., & Itoh, M. (2022). The guppy (Poecilia reticulata) is a useful model for analyzing age-dependent changes in metabolism, motor function, and gene expression. Experimental gerontology, 160, 111708. https://doi.org/10.1016/j.exger.2022.111708
Butler, J. M., & Maruska, K. P. (2015). The mechanosensory lateral line is used to assess opponents and mediate aggressive behaviors during territorial interactions in an African cichlid fish. The Journal of experimental biology, 218(Pt 20), 3284–3294. https://doi.org/10.1242/jeb.125948
Webb, J.F. and Shirey, J.E. (2003), Postembryonic development of the cranial lateral line canals and neuromasts in zebrafish. Dev. Dyn., 228:370385. https://doi.org/10.1002/dvdy.10385
Schwalbe, M. (2024). Lab 5 - Fish Lateral Line System Student Protocol.
Appendix
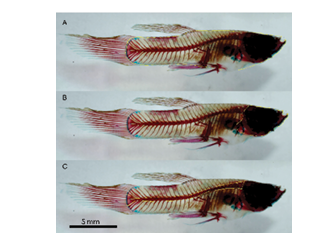 Figure 1: Measuring surface area of Poecilia reticulate in lateral view in ImageJ through tracing around the area of interest. Pictures show tracing of total surface (A), skull (B), and spine (C).
Figure 1: Measuring surface area of Poecilia reticulate in lateral view in ImageJ through tracing around the area of interest. Pictures show tracing of total surface (A), skull (B), and spine (C).

Figure 2: Photo of a set up for behavioral trials. The oscillating probe bead is placed in the water on the upper right side of the bucket. Desk lamp was used to provide light.
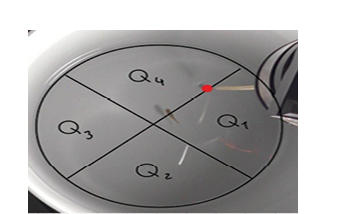
Figure 3: Annotated photo of a bucket from behavioral trials. The bucket is divided into four quadrant Q1-Q4. The oscillating probe is highlighted as a red dot between Q1 and Q4.


Figure 4: Fluorescent imaging of juvenile guppy. (A) Lateral View; (B) Dorsal View; (C) Ventral View. Canal neuromasts consisted of suborbital (SO), trunk (T), and infraorbital (IO). Body length: 1.5 ± 1 cm.

Figure 5: Fluorescence imaging of adult male guppy. (A) Lateral View; suborbital (SO), otic (OT), mandibular-pre-opercular (MD-PR), supratemporal (ST), trunk (T), and infraorbital (IO). (B) Dorsal View; Nares (N). (C) Ventral View. Body length: 2.2 ± 1 cm
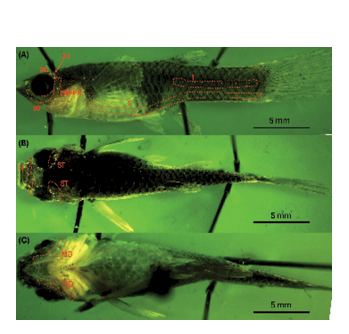
Figure 6: Fluorescence imaging of adult female guppy. (A) Lateral View; suborbital (SO), otic (OT), mandibular-pre-opercular (MD-PR), trunk (T), and infraorbital (IO). (B) Dorsal View; Nares (N). (C) Ventral View. Body length: 3.7 ± 1 cm.
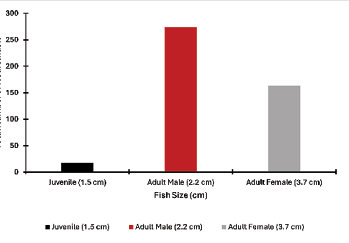
Figure 7: The total number of neuromasts among fish of different sizes. The largest number of neuromasts was on an adult male, followed by adult female and the juvenile. The neuromasts were counted from fluorescent images.
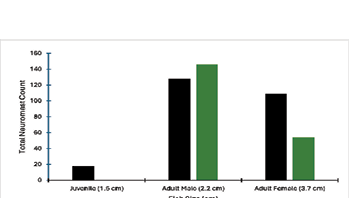
Figure 8: The proportion of canal and superficial nueromast in fish of different sizes. The largest number of both canal and superficial neuromasts was on an adult male, followed by adult female and a juvenile. The juvenile fih did not have any canal neuromasts. The neuromasts were counted from fluorescent images.
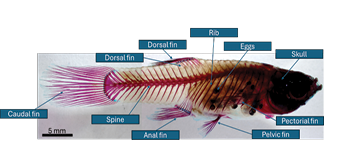
Figure 9: Labelled lateral view of the skeleton of 2.3 cm (largest) fish.
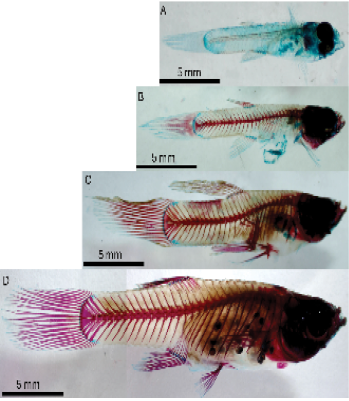
Figure 10: Lateral view of cleared and stained fish of 4 different lengths showing decreased cartilage (blue) and increased calcified bone (red) through growing fish lengths. 1.5cm fish (A), 1.7cm fish (B), 2.1cm fish (C), 2.3cm fish (D).
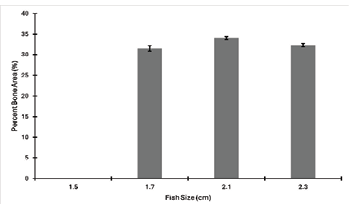
Figure 11: Percent calcified bone area as a function of fish size. Each fish area was measured in ImageJ three times and the average was used as actual the surface area. Error bars were calculated using standard deviations of the three area measurements.
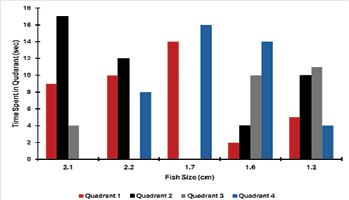
Figure 12: The data from behavioral trials is quantified as a time spent in each quadrant in seconds from a thirty second recording. The oscillating probe was set to 50 Hz at 10 V. In total, five fish were tested ranging from sizes of 2.1 cm to 1.2 cm.
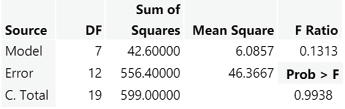
Figure 13: Analysis of variance ran for the behavioral trial using the JMP program. The results indicated no significant interaction between fish size and quadrant location, F(7) = 0.1313, p = 0.994.