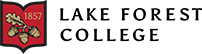Novel Insights into β-Synuclein and γ-Synuclein Toxicity in a Parkinson’s Disease Yeast Model
*This author wrote this paper as a senior thesis under the direction of Dr. Shubhik DebBurman
ABSTRACT
Synucleinopathies are diverse human neurodegenerative disorders linked to the misfolding and aggregation of proteins of the synuclein family. Unlike intense investigations with α-Synuclein, β- and γ-Synucleins remain understudied. Two β-Synuclein mutants (P123H and V70M) directly cause Dementia with Lewy Bodies (DLB), while γ-Synuclein is associated with Lou Gehrig’s disease. α-Synuclein pathogenicity is regulated by familial mutations, post-translational modifications, and several cellular environments altered in synucleinopathies. Here, I use a budding yeast (Saccharomyces cerevisiae) model to examine the hypothesis that β- and γ-Synucleins are differentially toxic and regulated by similar factors. I report that 1) High expression is key to β-Synuclein toxicity and aggregation; 2) Basic charge drives P123H-βS toxicity, while hydrophobicity underlies V70M-βS toxicity, and both mutants preserve α-Synuclein’s toxicity; 3) Amino acids A29, A30, G51, and A53 in β-Synuclein regulate its toxicity/aggregation, as does nitrative stress, reduced glycation, and increased acetylation; 4) γ-Synuclein is surprisingly non-toxic and cytoplasmically diffuse, and resistant to modifications, except when under nitrative
INTRODUCTION
Molecular Logic of Life’s Diversity
All living organisms, from the simplest single cell prokaryote to the most intricate eukaryotes, share the common instruction material vital for cellular functionality. This cornerstone of cellular processes is our genetic material known as DNA. DNA, or deoxyribonucleic acid, serves as the blueprint for proteins contributing to all diversity in life by storing information in a linear code made of four deoxyribonucleotides: Adenine, Thymine, Cytosine, and Guanine (Figure 1A). These nucleobases interact with each other giving DNA its specific structure of antiparallel double-helix. The central dogma delineates the flow of genetic information from DNA to RNA to the expression of traits through proteins (Ostrander, 2023). Additionally, the shape of the protein is what confers function (Figure 1B). As in the case of the proteasome, whose function is to break down and get rid of unwanted proteins, it has similar shape to what we know as a recycling bin (Figure 1B). To unravel the most fundamental organizational principles governing cells, we must conduct a thorough examination at the biochemical level, emphasizing the diverse array of mechanisms, structure, and chemical processes.
For this reason, our inquiry starts with DNA, which undergoes transcription within the nucleus, orchestrated by a multitude of enzymes, such as RNA polymerase III to give a complementary sequence of RNA. Subsequently, RNA leaves the nucleus and gets spliced to yield a mature messenger RNA (mRNA), ready to be translated into proteins at the ribosomes. The ribosomes employ another specialized type of RNA, transfer RNA (tRNA) to selectively bind to codons - three nucleotides coding sequence in the mRNA - to assemble a polypeptide chain comprised of amino acids (Ostrander, 2023). This entire process is interconnected, spanning from the genes that provide with the instructions for the linear sequence of amino acids, to the complex folding and acquisitions of a unique third-dimensional structure in functional proteins that allow for catalytic amino acids to react, see Figure 1 for a depiction of the central dogma.
DNA is susceptible to change
The central dogma controls the flow from our genes in the DNA, which provides instructions for the linear sequence of amino acids, to the folding and unique third-dimensional structure of proteins. The ability of proper and accurate folding determines the functionality of the protein (Figure 2A). Many situations can distort the shape of the protein, as in the case of mutations. As described before, DNA is the blueprint to build proteins, however, DNA is susceptible to change, and these changes are called mutations (Figure 2A). Despite their negative connotations, mutations are not necessarily bad at times. Changes can vary from a single nucleotide insertion to a complete addition of a gene, such as in the case of an opsin gene, which helps primate have an extended and expanded range of color vision (Neitz and Neitz, 2011). This example demonstrates how mutations can be beneficial for the organism. Further studies showed that mutations are also linked to evolution, driving survival and reproduction. For example, antibiotic resistance in bacteria results from the acquisition of multiple copies and changes in protective genes that confer an antibiotic resistance phenotype to bacteria (Martinez and Baquero, 2000).
On the other hand, mutations can also be neutral and detrimental. Heterochromia is a genetically acquired condition characterized by differences in the iris coloration. Mutations in the DNA during cell division result in this condition; however, heterochromia is considered rather harmless, and the eye color causes no differences in the visual system (Stokkermans and Lui, 2023). In contrast, some genetic alterations, like the one in Huntington’s Disease (HD), can lead to harmful disorders. In HD, a mutation occurs in the gene coding for the protein Huntingtin,
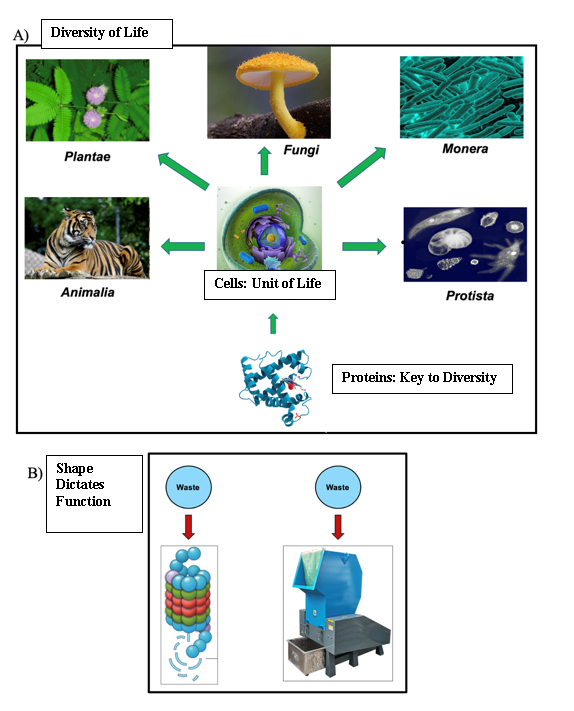
Figure 1. Proteins are the key to life’s diversity. A. All five kingdoms show extraordinary diversity on Earth. All these organisms are composed of cells, and within them, they have proteins. Proteins confer the great diversity on the planet. B. Shapes of proteins dictate function. For a protein to be in its healthy state it takes on a three-dimensional structure by folding. This shape is important for function and identify of the protein.
increasing the number of CAG repeats from its normal range of 27 to 35, to 36 or more repeats, consequently synthesizing an abnormally long protein and leading to neuronal degeneration (Jiang et al., 2023). All these conditions highlight and emphasize the extensive variability caused by genetic mutations, and why I find the study of the cause of these genetic mutations’ imperative.
Tracing Origins of Disease: Loss of Original Amino Acid
Interestingly, sometimes it only takes a seemingly subtle change, the loss of the original amino acid in a gene mutation, to trigger a chain of significant and detrimental consequences like protein toxicity. This change is evident in the case of sickle cell anemia (Figure 2B). The loss of glutamic acid in position 6 of the beta-globin chain, replaced by valine, causes this disease, by making the sickle cell hemoglobin (hemoglobin S) have an extra hydrophobic residue (valine) on the surface of the protein (Nelson & Cox, 2017). This leads to deoxygenated hemoglobin S to become insoluble and polymerize into aggregation, producing the elongated, rigid shape, and consequently, a changed of function of the (Steinberg, 1998). When examining the molecular structure of each of these amino acids, we observe that glutamic acid – which is negatively charged - is a polar residue with the capability to participate in hydrogen bonding. In contrast, valine is a nonpolar amino acid containing only carbon and hydrogen. The switch from being acidic to being hydrophobic, aiding and causing aggregation, produces the detrimental changes observed in the loss of structure underlying the disease (Nelson & Cox, 2017).
Tracing Origins of Disease: Gain of New Amino Acid
Another explanation for protein toxicity is the result of gaining a particular amino acid through a gene mutation (Figure 2C). This is the case with the autosomal dominant condition
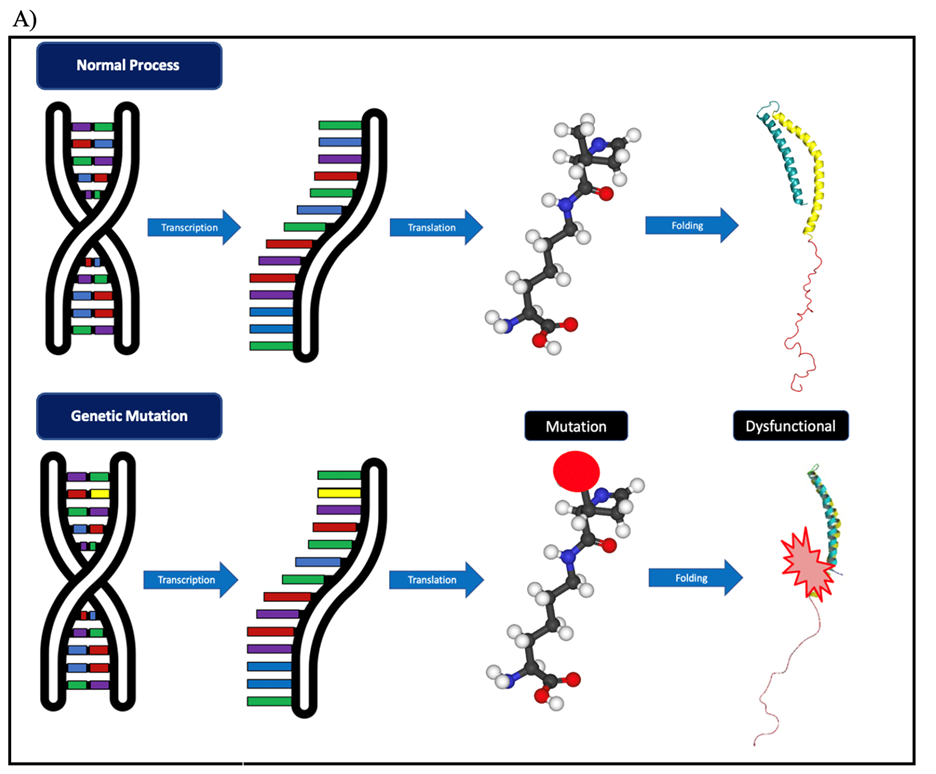
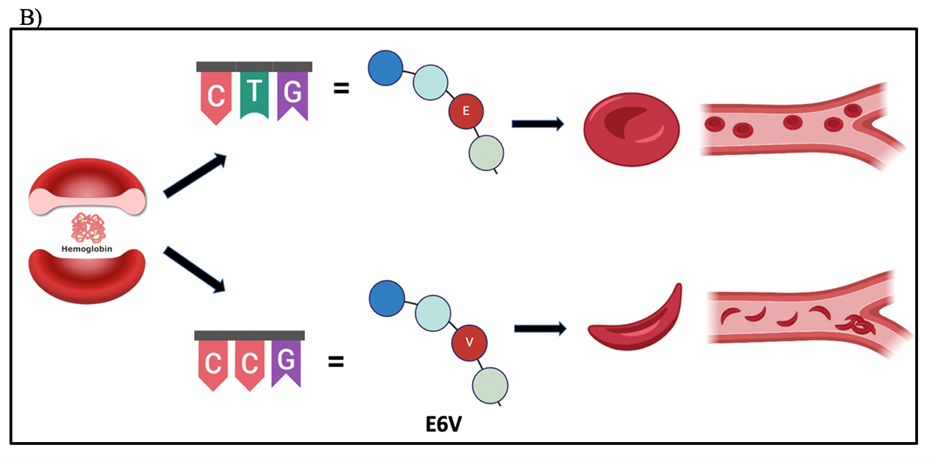
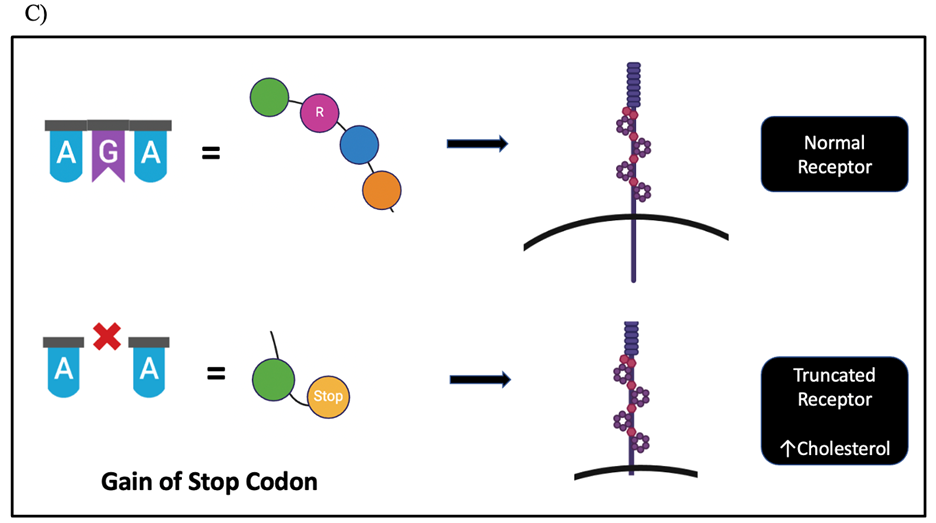
Figure 2. Mutations in amino acid sequences. A) Central Dogma of Biology. B) Loss of original amino acid led to sickle cells changing the structure of red blood cells, and thus, producing hemoglobin to form inflexible chains. C) When gain of new amino acid due to deletion mutation can lead to premature stop codon, truncating the protein, and thus rendering the LDLR receptor unfunctional. Their DNA sequence will have 5’ to 3’ ends for each.
Familial Hypercholesterolemia (FH). Its pathology comprises a high concentration of low-density lipoprotein cholesterol (LDL-C) in the bloodstream and the inability to remove it from the blood.
The reason for the change is a mutation in the LDL receptor gene, which is responsible for metabolizing the “bad” cholesterol in the bloodstream (Callis et al., 1998). Researchers discovered a novel G deletion at codon 185 on chromosome 19 of the LDL receptor gene. This deletion leads to a gain of a premature stop codon, resulting in a truncated receptor that impairs the regulation of cholesterol. The accumulation of unwanted cholesterol is strongly linked to the buildup of fatty acid deposits in the blood vessels, and associated with cardiovascular diseases (Callis et al., 1998). This thesis delves into studying further changes due to the absence or presence of a single amino acid. Understanding the intricate interplay between mutations and protein dysfunction is key to shedding light on the molecular mechanisms of toxicity (Figure 2A).
Neurodegenerative Disease
Neurodegenerative diseases (NDDs) are the group of disorders characterized by the misfolding and aggregation of proteins in the nervous system (Wilson et al., 2023). As the name indicates, the progressive neuronal death overtime causes the pathological aggregation of proteins. These are two out of eight hallmarks of NDDs, which also include: inflammation, synaptic and neuronal network dysfunction, altered energy homeostasis, DNA and RNA defects, cytoskeletal abnormalities, and aberrant proteostasis (Wilson et al., 2023). Even though all NDDs share the same hallmarks, the symptoms are linked to which protein is aggregated and the region in which is located. For example, AD, the most prevalent neurodegenerative disease, pathology is amyloid plaques of aggregate β-amyloid and neurofibrillary tangles of tau protein found in the cholinergic neurons in the hippocampus and amygdala, among other limbic areas (Selkoe & Lansbury, 1999; Wilson et al., 2023). In AD symptoms, a deterioration to these structures exists, which are the areas controlling and regulating social cognition, long-term, spatial, and emotional memory (Rajmohan, & Mohandas, 2007). HD arises from the expansion of the HTT gene, resulting in a gain of function for the huntingtin protein (Jiang et al., 2023). These CAG repeats induce the accumulation of HTT protein and subsequent degeneration of the putamen and caudate nucleus. These structures play pivotal roles in processes such as decision-making, reward processing, and motor control, all of which manifest behavioral impairments in individuals afflicted with HD (Jiang et al., 2023). This thesis will focus on a specific subset of NDDs, referred to as Synucleinopathies, characterized by the shared pathology of α-Synuclein (αS) protein aggregation.
Synucleinopathies
The Synucleinopathies constitute a subset of NDDs distinguished by the presence of aggregated αS in neuronal and/or glial inclusions (Koga et al., 2021). They are categorized into two primary disease entities: MSA and Lewy body disease, which encompass DLB and PD (Koga et al., 2021; Figure 3). All these diseases demonstrate the presence of Lewy Bodies (LBs), which are round inclusions mostly composed of aggregated αS, as well as some damaged mitochondria, lysosomes, and vesicles (Koga et al., 2021). They are found in neuronal and glial cell bodies and neurites, such as the dendrites and axons. MSA, is a rare, but fatal sporadic NDD that primarily affects oligodendrocytes, which oversee producing myelin in the brain and spinal cord (Flabeau et al., 2010). The two subtypes of MSA are Parkinsonian type (MSA-P) with striatonigral degeneration pathology and Cerebellar type (MSA-C) characterized by olivopontocerebellar (olivary nuclei, cerebellar peduncles, and cerebellum) atrophy (Jellinger & Wenning, 2005). These classifications separate according to their symptomatology, having MSA-P manifest Parkinsonism with rigidity, bradykinesia, and abnormalities in the autonomic nervous system, whereas MSA-C displays more ataxic symptoms, marked by challenges in coordination and balance (Flabeau et al., 2010). Furthermore, cerebral dysfunction is also observed more in MSA-C rather than in MSA-P. Differentiation between MSA-C and MSA-P is not always clear, they have overlapping. This is attributed to the shared core pathology, namely the progressive loss of oligodendrocytes causing a decrease of neuronal myelination, thereby degrading myelin sheath, and affecting neuronal functioning (Flabeau et al., 2010).
Another synucleinopathy is DLB, also a progressive NDD affecting emotional and thinking patterns to behavioral and movement coordination. Similarly, to MSA, DLB has Lewy bodies, but also shows evidence for Lewy neurites (LNs), which are the misfolded and aggregated αS forming abnormal filamentous inclusions in dendrites and axons. DLB shows similar pathology to AD, having in some cases β-amyloid plaques, and having a 29-89% of cases being able to be diagnosed for both DLB and AD (Koga et al., 2021). DLB consists of cognitive symptoms, such as visual hallucinations, alertness, and loss of logical and organized thinking, movement symptoms (bradykinesia), and psychological symptoms like depression, anxiety, and restlessness (National Institute on Aging, 2021). Moreover, DLB Lewy bodies are in different areas of the brain having a higher density in the substantia nigra, followed by the entorhinal cortex, then the cingulate gyrus, insula, frontal cortex, hippocampus, and occipital cortex, respectively (Gómez-Tortosa et al., 1999). DLB clinical diagnosis is further challenged by the similarities and associations to PD.
PD, the second most prevalent NDD after AD, affecting more than 10 million people worldwide (Parkinson’s Foundation, n.d.). As a global source of disability, the fast increase in mortality rate and prevalence led researchers declare a Parkinson Pandemic (non-infectious) based on the doubling of cases in the last decades, with expected cases increasing over 12 million by
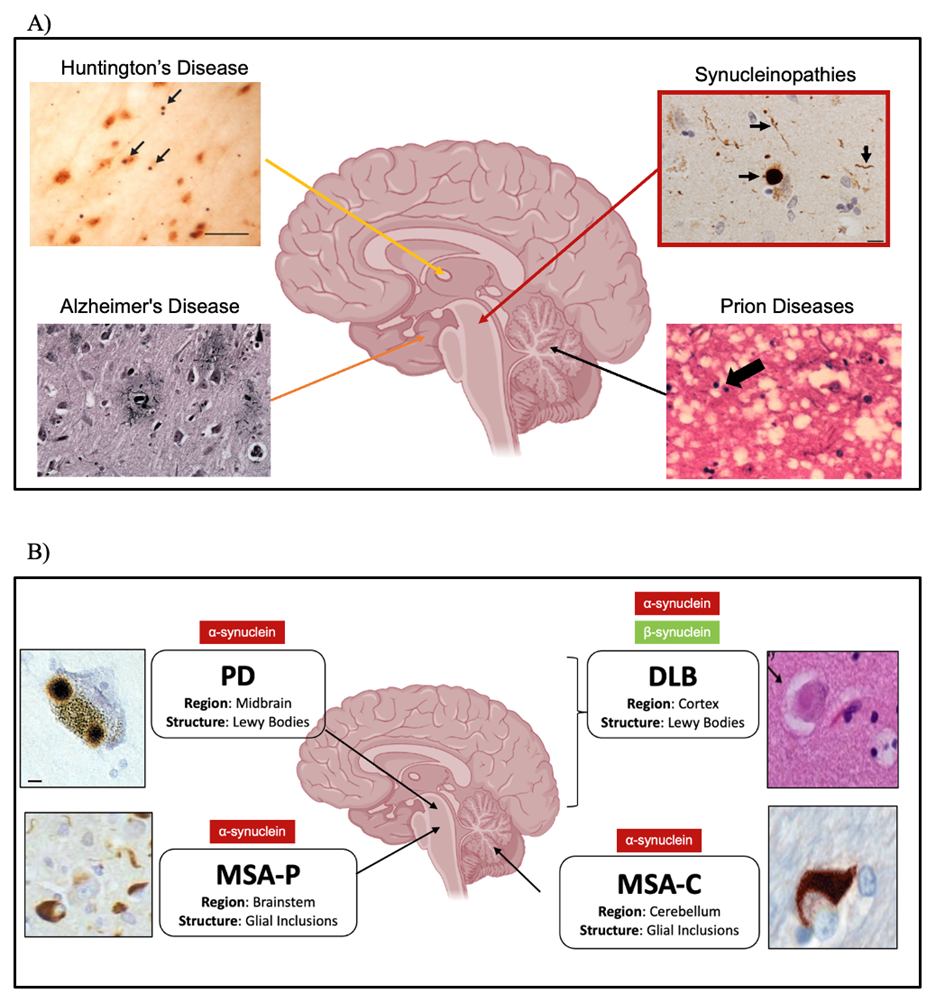
Figure 3. Neurodegenerative Disease. A) Neurodegenerative diseases are characterized by the misfolding and aggregation of specific proteins within the brain. Some of the most known and studied ones are AD, HD, PD, and prion disease. The former one is characterized by the progressive cell death of memory cells, whereas the latter three are characterized by the neuronal death of movements cells. The cells that get affected are linked with the types of symptoms, and thus, why in AD is linked to memory loss. For the other three movement regulation problems. B) Synucleinopathies are NDD that their pathology consists of misfolding of the synucleins, specifically of aS in all of them. βS mutants in DLB. Depending on where the misfolding and aggregation is found, it leads to the diagnosis of the different synucleinopathies.
2040 (Dorsey et al., 2018). It has a variable age of onset, but like most NDDs, typically present symptoms in mid-to-late adulthood, with no known cure. In terms of treatments, the emphasis lies in enhancing quality of life by mitigating motor and non-motor symptoms. The former, often attributed to PD due to its relatively more apparent and faster manifestation, encompasses the following cardinal symptoms: Rigidity, bradykinesia (slowness of movement), postural instability, and resting tremors (Dickson, 2012). The latter, non-motor symptoms, are equally significant and encompass factors such as depression, visual and auditory hallucinations, impaired communication, and sleep disturbances, among others (Dickson, 2012).
While PD presents a diverse range of symptoms, the condition initiates its development much earlier, often in an asymptomatic state. The absence of discernible symptoms prior to onset adds an additional layer of complexity to the challenging diagnostic process for PD. Most patients’ symptoms express later in the disease, doctors find a significant decline in striatal dopamine (DA) and substantia nigra pars compacta (SNc) neurons in patients with 70%-80% and almost a third of each of these, respectively, already lost by the time symptoms appear (Wu et al., 2011). Even until today, PD is not studied clinically using a neuropathological method, the closest invention is the Dopamine Transporter Imaging (DaTscan). The DaTscan, a 2011 FDA-approved imaging technique uses a radioactive isotope (Ioflupane-123) injected to the patient veins, that circulates through the bloodstream until uptake by dopamine transporters – the radiotracer is designed to bind specifically to these in the brain. Using a single-photon emission computed tomography scanner (SPECT scanner) a doctor is capable to visualize dopamine transporters in the brain and assess the condition of the dopamine system in the nigrostriatal pathway. PD patients show evidence for an abnormal image as a result of the loss of dopamine-producing neurons (Catafau & Tolosa, 2004). Overall, this technique is accompanied by the patients’ medical and symptom-history records by trying to aid in better clinical diagnosis.
PD Causes: Familiar and Sporadic Types
Looking more in depth to PD, the neuropathology is characterized by the misfolding and aggregation of αS forming LBs in the dopaminergic neurons of the SNc in the midbrain, leading to neuronal death. Therefore, producing a reduction in dopamine in the dopaminergic projections from the SNc to the striatum (structure in the basal ganglia), all referred as the nigrostriatal pathway (Sonne et al., 2023). PD can be both monogenic and environmentally determined, contributing to familiar and sporadic PD types (Figure 4A). Starting with sporadic, even though less understood sporadic PD consists of 80-90% of cases. Sporadic PD is more aggressive and has a later onset as compared to familiar PD (Inzelberg et al., 2004). Furthermore, Sporadic PD is the consequence of environmental stressors such as high levels of air pollutants like nitrogen dioxide – from combustion of fossil fuels - as a risk in PD development (Jo et al., 2021; Ritz et al., 2012), exposure to places with large use of pesticides, multiple studies have recorded a few that increase the risks including paraquat, dieldrin, and rotenone (Breckenridge et al., 2016; Pouchieu et al., 2018; Tangamornsuksan et al., 2019), even mining workers are considered at possible risk due to exposure to manganese, a heavy metals (Gorell et al., 1997; Racette et al., 2017). Environmental stressors do not operate in isolation either. Studies using genome-wide association assays in PD researchers found that αS-encoding gene locus, SNCA, polymorphisms contribute to Sporadic PD (Nalls et al., 2014), as well as random mutations (Kasten & Klein, 2013; Figure 4C).
Familiar PD was established with the discovery of an inherited cause for PD, supported by an autosomal dominant mutation in the SNCA gene coding for αS in three independent Greek families and an Italian family (Polymeropoulos et al., 1997; Figure 4B). Multiple other genes now linked with PD, such as LRRK2, also autosomal dominant, PARK2, PINK1, and DJ-1 are autosomal recessive mutations (Konovalova et al., 2015). Lastly, the GBA gene is linked to PD, but it does not follow the conventional Mendelian inheritance, for this the mutation ought to cause a loss-of-function, usually in the case of autosomal-recessive (two alleles needed carrying mutation), and a gain-of function for autosomal dominant (one allele needed carrying mutation). However, GBA produces a gain and a loss-of-function in parkinsonism (Sidransky & Lopez, 2012).
Synucleinopathies: A Family Tale
The current literature provides substantial compilation of information pertaining to αS and its role in synucleinopathies; however, αS forms part of a larger synuclein family. This family is composed of αS, β-Synuclein (βS), and g-Synuclein (𝛾S). All of these proteins are homologs with a considerably similar flexible structure with high levels of evolutionary conservation in the nervous system, and they are only found in vertebrates (Surguchev & Surguchov, 2017). αS is the longest out of the three, consisting of 140 amino acids, followed by βS with 134 amino acids, and 𝛾S having 127 amino acids; the proteins get made by 5 coding exons that are similar in all three. Their evolutionary conserved sequence indicates a significant role of these proteins that in the case of αS and βS share 61% similarities, and with 𝛾S 55.9% similarities (Lavedan, 1998); this homology increases if the focus is the amino terminal which has 76% similarities between αS and βS, and 67% with αS and 𝛾S (Burré et al., 2018; Jakes et al., 1994). Refer to Figure 4 for a comparison between αS, βS, and 𝛾S amino acid sequence alignment.
a-Synuclein Protein
αS encoded by the SNCA gene, is a small, flexible, yet abundant presynaptic protein within the nervous system (Figure 4B). αS is present in the central and peripheral nervous system involved in neuronal function, with high concentrations found in the substantia nigra, thalamus, hippocampus, neocortex, and cerebellum (Emamzadeh, 2016). The N-terminus (amino acid 1 to 60) has membrane-binding capabilities because of its amphipathic properties. The KTKEGV motifs are seven repeats of 11 residues that encompass a combination of acidic, basic, polar, and nonpolar amino acids (Dettmer et al., 2015). Notably, this motif is unique to the synuclein family, despite being highly conserved across all three synucleins. Although many studies using techniques like NMR spectroscopy, X-ray fiber diffraction, and EM reconstruction identified the protein’s native state, most biochemical assays indicate a combination of α-helical structures formation (Eliezer et al., 2001; Ulmer et al., 2005; Wang et al., 2011), disorder after dissociation from the cell membrane (Fauvet et al., 2012), and various combinations of both (Burré et al., 2013).
Conversely, the NAC domain (amino acids 61-95) exhibits higher hydrophobicity compared to the other two domains, and it also encompasses the previously described unique motif (Burré et al., 2018; Figure 5A). This region facilitates aggregation by promoting protein polymerization (Burré et al., 2018). Lastly, the negatively charged carboxy-terminus (C-terminus), spanning from amino acid 96 to the last 140, has many polar residues. The C-terminus contains the least conserved sequences among the synucleins, and its function is not as well understood as the others. Nevertheless, within this domain, αS undergoes various post-translational modifications, including phosphorylation (Fujiwara et al., 2002), glycation (Padmaraju et al., 2011), ubiquitination (Nonaka et al., 2005), acetylation (Maltsev et al., 2012; Dikiy & Eliezer, 2014), nitration (Giasson et al., 2000), among others. Some of these modifications serve a protective role against toxicity, as in the case of the first three, while others induce structural changes and altered functionality. In the case of nitration, some of the toxicity observed in αS is attributed to the change of properties due to the addition of the nitro group. This chemical group gets added to the tyrosine residues in αS (Y39, Y125, Y133, Y136, and others) and the change causes a shift on the residues’ pKa from their normal 10 to 7.50 (Corpas et al., 2009; Sano et al., 2021). Additionally, the nitro group presents steric hindrance, affecting the charge distribution, and the bonding with other residues, since tyrosine is a fundamental amino acid for aromatic stacking and hydrogen bonding necessary for protein folding (Sano et al., 2021). These alterations to the protein structure can influence the propensity of αS to polymerize and aggregate, and thus, why higher nitration is categorized as an enhance or αS toxicity.
The role of αS is still being elucidated, with a common consensus that αS is involved in vesicle budding with presynaptic membrane, aiding in neurotransmitter release, and thus, in synaptic trafficking (Chandra et al., 2003; Varkey et al., 2010; Sharma & Burré, 2023). αS is repeatedly linked with NDDs because of its NAC domain tendency to adopt a β-sheet conformation, promoting misfolded αS to form fibrils and aggregate (Tuttle et al., 2016). These fibrils are insoluble, and the ones found primarily in LBs. Moreover, through the aggregation process of this protein, αS changes from monomeric unit to form small oligomers that then become and grow into fibrils (Goldberg & Lansbury, 2000; Cookson, 2009).
α-Synuclein Mutations
The synucleinopathies pathology of αS fibrils in LBs needs to be biochemically described by the molecular interaction of the protein. The structure of αS fibrils is established due to the salt-bridges, H-bonding, and steric zippers that form and can be elicited by mutations in the SNCA gene. Six old known familiar mutations are highly implicated in aggressive early onset PD. All of these are in the amphipathic N-terminus suggesting the importance of membrane-binding from this domain on αS toxicity.
These six mutants associated with familiar PD are: A30P, E46K, Q50H, G51D, A53E, and A53T (Figure 4C). As well as three newer mutants that are linked with novel familiar PD – A53V – and sporadic PD – A18T and A29S. In the lab, we focus on all these mutants compared them to wildtype (WT) forms of the synucleins and analyze their recipe for toxicity.
Older mutants
A53T
The first mutation in αS’ sequence to be discovered was A53T. Researchers identified it in 1997 in a Greek family and using GWAS, in other three Greek unrelated families, Sicilian kindred, in a Swedish, and Korean family (Whittaker et al., 2017). This mutation involves the change from the nonpolar (hydrophobic) amino acid alanine in position 53 to the uncharged polar, larger amino acid threonine. This mutation is an autosomal dominant mutation that gives early onset PD, with an average age of 48 years old (Whittaker et al., 2017). When the mutation A53T is present in αS in transgenic mice, the protein binds to the membrane similarly to αS (Sharon et al., 2001) and in budding and fission yeast (Brandis et al., 2006; Sharma et al., 2006).
A30P
The second mutation discovered is A30P in a German family during 1998 (Whittaker et al., 2017). The mutation involves a change from nonpolar (hydrophobic) amino acid alanine in position 30th to another nonpolar (less hydrophobic) cyclic amino acid proline. In contrast with A53T, A30P αS’s toxicity is not explained by aggregation. A30P in αS makes the protein not bind to the membrane when present, it stays diffuse in the cytosol (Jensen et al., 1998).
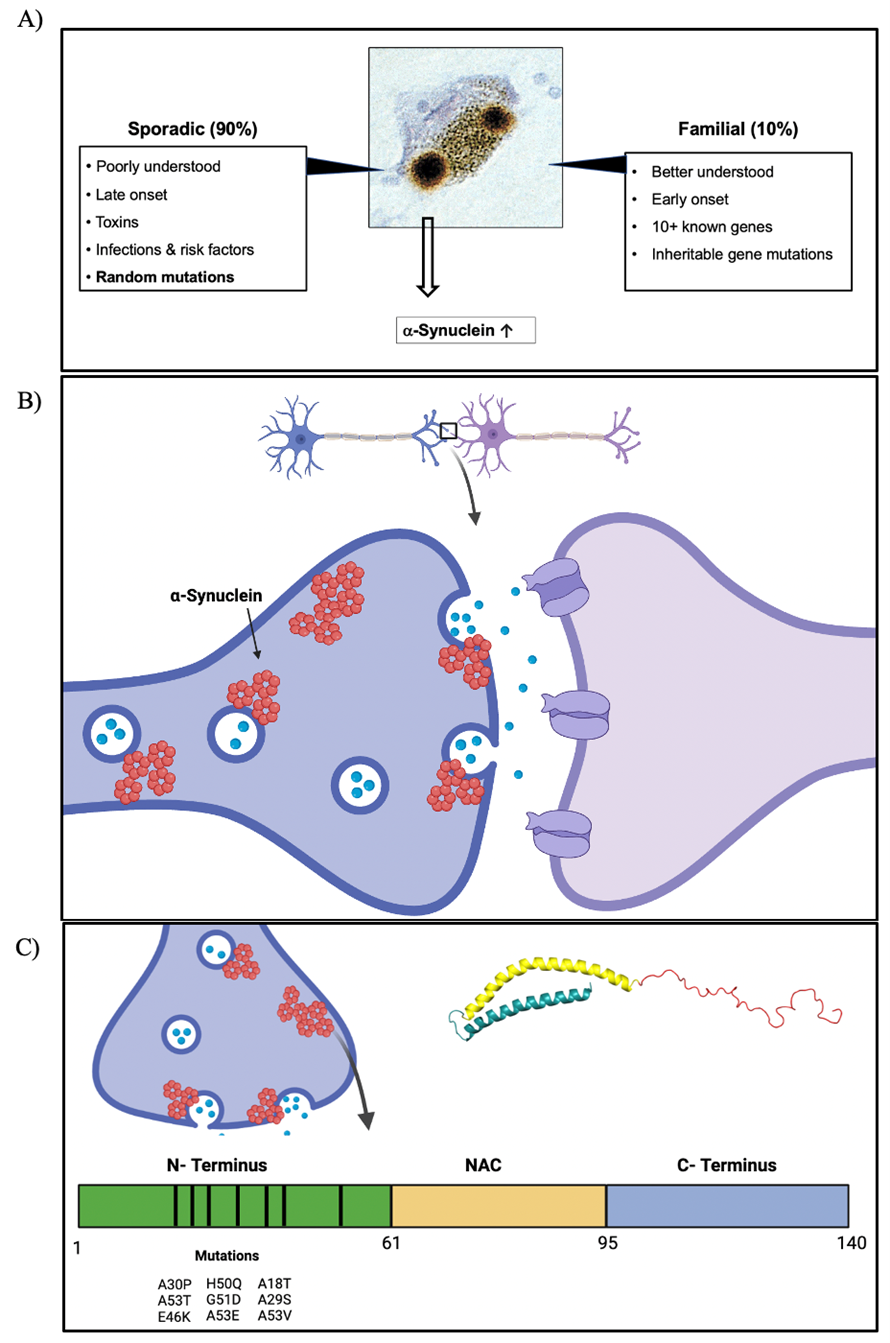
Figure 4. Molecular Biology of PD. A) PD is categorized into two types, one is sporadic (90% of cases) and the other is familiar (10% of cases). B) aS pathology. aS is a synaptic protein that is commonly found helping in vesicle trafficking, regulation, and SNARE complex fusion with membrane. C) aS protein has three domains, N-terminus (amphipathic domain linked with lipid and membrane binding properties), NAC region (hydrophobic domain linked with aggregation), and C-terminus (Acidic domain regulating solubility). The nine known mutations from early-onset PD patients occur in the N-terminus. These are A18T, A29S, A30P, E46K, Q50H, G51D, A53T/E/V.
Moreover, studies of A30P mutation using fluorescence resonance energy transfer showed an induced conformational change of αS preventing it from forming its secondary α-helix structure (McLean et al, 2000; Jo et al., 2002). This alteration shows the dominance of the proline, which has a cyclic structure impairing the correct folding and membrane-binding ability, rendering the protein more prone to misfolding which is a hallmark of PD. The two different mutations A30P and A53T both contribute to early onset PD but through different unique mechanisms.
E46K
The next mutation to be discovered was E46K, changing the negatively charged (acidic) glutamic acid to a positively charged (basic) lysine at the 46th position. This mutation was found in a Basque Country family in 2004 with severe neuronal loss in the substantia nigra, locus coeruleus, and dorsal nuclei of the vagus (Whittaker et al., 2017). The mutation’s position is in the KTKEGV motifs mentioned before. In vitro studies have revealed that with this mutation, αS forms and polymerizes into insoluble fibrils quicker than αS, and these fibrils are like amyloid fibrils similar to the ones that form in presence of the A53T mutation (Greenbaum et al., 2005; Fredenburg et al., 2007). Furthermore, αS shows high affinity for liposomes and phospholipid binding (Choi et al, 2004), this property combined with the high propensity to polymerize are the cause of toxicity for the E46K mutation.
H50Q, G51D, A53E
The less characterized familial mutants of αS are H50Q, G51D, and A53E. These were discovered in an English and a Canadian patient (Appel-Cresswe et al., 2013), a French patient (Lesage et al, 2013), and a Finish patient (Pasanen et al., 2014), respectively. The H50Q involves a change in the basic amino acid histidine for the polar amino acid glutamine in the 50th site. H50Q- αS has faster aggregation than αS due to having two forms of protofilaments as compared only one (Boyer et al., 2019). H50Q-αS has faster fibril formation activity as compared to all other mutations, promoting, and accelerating αS aggregation (Guan, et al., 2020).
G51D involves the change from the nonpolar Glycine amino acid in position 51st to the negatively charged (acidic) amino acid aspartic acid. And A53E involves the change from nonpolar (hydrophobic) alanine to acidic glutamic acid in position 53rd. G51D with A53E and A53T give rise to the earliest age of onset of PD, but contrary to A53T, G51D and A53E are similar to A30P in which they reduce αS aggregation and binding to the membrane (Goedert et al., 2017). Literature shows these mutants such as G51D are present with non-toxic traits, however, contribute to the misfolding and aggregation of αS (Fares et al., 2014). One explanation is other modifications such as post-translational/covalent modifications in specific sites or altered cellular environments (Fares et al., 2014).
Newer mutants
A18T, A29S, and A53V
These two sporadic mutations were each identified in a Polish family and linked have different onsets, with A18T showing an earlier onset of symptoms than A29S (Hoffman- Zacharska et al., 2013). And A53T was identified in a Japanese patient with late onset PD in an autosomal dominant inheritance (Yoshino et al., 2017). These genetic mutations are fairly new, and thus, heavily understudied. Our lab started assessing if these genetic mutations are toxic in yeast which led to two senior theses in the last three years by Carris Borland ’21 and Amanda Grassel ’23, who I helped collecting data for. They showed the three newer mutants are less toxic than αS in yeast, however, many factors and modifications regulate their toxicity. Grassel reported that to unlock the newer mutants’ toxicity, they needed different recipes. She saw that A18T and A53V were particularly sensitive and more toxic with high levels of nitrative stress, whereas A29S was more sensitive in environments where specific PD genes were knocked out, and sensitive to altered SUMOylation levels (Grassel, Thesis, 2023). Even though these genetic mutations need further work, Borland and Grassel started assessing and characterizing them in yeast.
β-Synuclein Protein in NDDs
The second member of the synuclein family is βS consisting of 134 amino acids and coded for by the SNCB gene. Like αS, βS has an N-terminus expanding from amino acid M1 to K60, an NAC domain (E61-V95), and a C-terminus (K96-A134). Its sequence is high in number of the nonpolar amino acid alanine making 13.4% of the sequence, and the acidic glutamic acid forming 18.7% of its sequence; and contains no cysteine nor tryptophan residues (Hayashi & Carver, 2022a). The body expresses βS in the central nervous system, specifically in astrocytes, myelin, olfactory epithelium, skeletal muscle, and basal forebrain (Hayashi & Carver, 2022a). Unlike αS, βS is slightly dispersed in the substantia nigra and striatum (Li et al., 2002). The biggest difference in sequence between βS and αS is the deletion in the NAC region of amino acids 53-63, which is the domain related to aggregation (Figure 5B). This deletion of a piece of the NAC region led to researchers believe βS to be unable to form the fibrils seen in LBs in a variety of physiological conditions (Uversky et al., 2002; Leitao et al., 2018; Van de Vondel et al., 2018). Researchers believed βS to be nontoxic and protective against αS toxicity. However, multiple recent studies report that βS protective role is not entirely true. βS shows a high degree of intrinsically disordered structure, capable of changing and adapting conformation to the necessary function. Using a vibrational spectroscopic technique called Raman optical activity (ROA), researchers were able to show that βS undergoes a conformation change to take on a polyproline type-II (PP-II) helical conformation, and that this conformation can serve as a template for amyloid fibrils (Syme et al., 2002).
Since βS has a disordered structure, it has many distinct functions within the cells. For example, βS is implicated in lipid binding, similar to αS. Since βS has five conserved repeats of the KTKEGV motif, such as those in class apolipoproteins A2, suggesting lipoprotein lipase activity regulation (Sharon et al., 2001; Westphal & Chandra, 2013). Despite its disordered structure, βS can do this function by changing and rearranging its conformation when in contact with lipoproteins to adopt an α-helical shape (Brown et al., 2016). Furthermore, based on its ability to bind to lipids with high affinity, βS regulates synaptic functioning and is in charge of neurotransmitter release, specifically of the Nigrostriatal Dopaminergic System. βS is found to be necessary for dopamine regulation in the projecting axons from the substantia nigra to the dorsal striatum. One group of researchers discovered using mice that those that lack βS had significantly less dopamine uptake by the vesicular monoamine transporter-2 (VMAT-2; Ninkina et al., 2021). Another study done in transgenic mice reported a different outcome of lack of βS. They saw those mice lacking βS had symptoms of motor discoordination, decreased sensorimotor function, and strength (Synucleinopathies-like symptoms) as compared to WT, providing more evidence to support the claim that βS aids in the regulation of DA uptake (Connor-Robson et al., 2016).
β-Synuclein Mutations in DLB
Recent studies report two βS missense mutations in the SNCB, V70M and P123H, that are implicated in NDDs (Figure 5B). The former one was first reported by Ohtake et al. (2004) in an 83-year-old Japanese man that had DLB. This genetic mutation was found in the NAC region and produced a change from the hydrophilic amino acid valine in position 70th to another less hydrophobic amino acid methionine. Researchers believe this change involves a substitution of an amino acid with a residual branch to a linear sulfur-containing amino acid. The presence of sulfur and the methyl group allows formation of a thioether functional group which can contribute to conformational changes and low solubility. This change in amino acid is believed to have created the conformational changes seen in pathological condition of βS. Researchers used transfected rat neuroblast cells with βS-V70M to analyze pathology and found aggregations and inclusions in the cytosol while using markers for lysosomal dysfunction (e.g.: Cathepsin and BLAMP-2; Wei et al., 2007). Recently, studies done using yeast expressing βS-V70M shows double the percentage of yeast with inclusions as compared to βS demonstrating a significant molecular effect of this mutation in pathology, specifically related to impairments in the trafficking of vesicles and lysosomal impairments (Tenreiro et al., 2016).
The second genetic mutation, βS-P123H involves the substitution in position 123 of proline, a rigid cyclic amino acid, to histidine, a basic, hydrophilic amino acid. It was found in a familiar autosomal dominant case of DLB. Similarly, using transgenic mice, βS-P123H can form insoluble aggregates in cytosol in vitro with lysosomal dysfunction pathology (Fujita et al., 2010). Expression of βS-P123H also led to axonal swelling, dependent on age, and astrogliosis – reaction of astrocytes to neuronal damage - in the globus pallidus and striatum (Fujita et al., 2010). Mice also presented cognitive deficits investigated by behavioral tests using the water maze and target quadrant tests for memory and learning, which were both significantly lower as compared to non-tg mice; this was followed by motor deficits after six months. Furthermore, using immunohistochemistry it was found that 14-months-old transgenic mice expressing βS-P123H had a significant decrease of biomarkers involved with mature granule cells, and a high expression of biomarkers related to immature granule cells, microglia, and neuroinflammation in the dentate
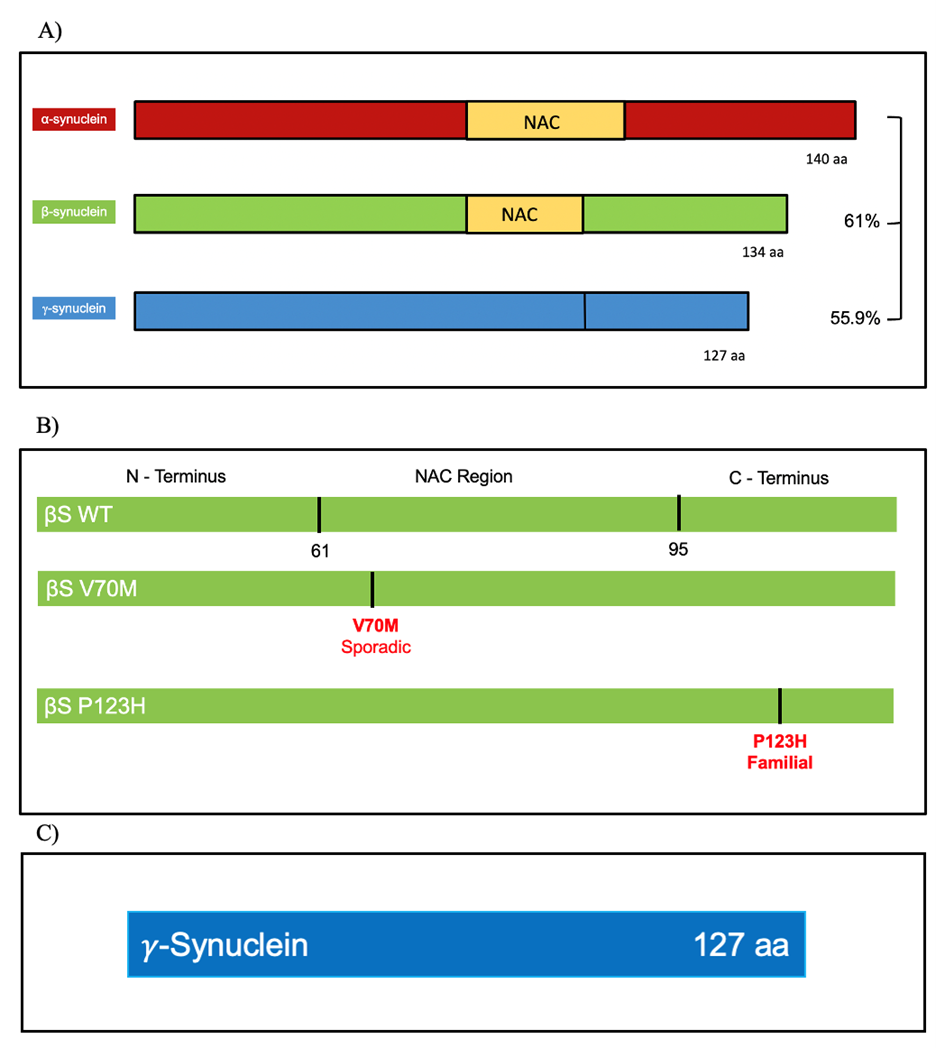
Figure 5. Comparison between aS, bS, and gS proteins. a) Sequence homology between the synuclein protein family, percentages are in relation to aS. b) βS sequence and the two known mutants. c) gS sequence, it does not contain an NAC region.
gyrus of the hippocampus (Hagihara et al., 2018). All of these studies provide a gain-of-function mechanism of toxicity produced by βS-P123H.
To understand the difference in toxicity potential between βS and βS-P123H biochemists looked at their conformational structure by using heteronuclear single quantum coherence profiles (HSQC) and electrospray ionization mass spectrometry (ES-IMS). HSQC analysis yielded these two proteins remarkably similar structurally, but βS-P123H shows more compact conformational ensembles than βS (Janowska & Baum, 2016); and βS-P123H has more conformation similarities to αS as compared to βS in terms of the distribution of population of conformations. αS showed to have compact conformation 49% and extended 51%, while βS-P123H showed 51% compact conformation and 49% extended, and βS with the lowest similarity of 46% being compact and 54% extended (Janowska & Baum, 2016). These differences are caused by the changes in the C-terminus of each protein. As stated, the C-terminus is the least conserved between the synuclein families, and the P123H substitution is found in this domain. Furthermore, sequence analysis shows a repetition of proline in amino acids 122 and 123 (mutation site), not found in αS (P122, S123). The loss of the second proline provides a much more flexible C-terminus, triggering self-aggregation, and the gain of P123H allows H-bonding properties similar to the interactions of αS S123 (Janowska & Baum, 2016).
𝛾-Synuclein in NDDs
The smallest protein in the synuclein family is γS with 127 amino acids, and no NAC domain (Figure 5C). γS is expressed by the gene SNCG, mostly in the peripheral nervous system, in glia and in dopaminergic neurons in the substantia nigra, red nucleus, and anterior commissure (Brenz Verca et al., 2003; Galvin et al., 20011). γS is not highly implicated in synucleinopathies, however, novel studies with transgenic mice show γS accumulation into inclusions leads to the hallmarks of amyotrophic lateral sclerosis (ALS) patients producing cell death and motor dysfunction symptoms (Peters et al., 2012). ALS is a progressive fatal neurodegenerative disease characterized by motor neuron death weaking and stiffing muscles, distorting speech patterns, and more (Peters et al., 2012). Similarly, to αS, γS is naturally unfolded but in solution it can rapidly form and self-associate into oligomers (Golebiewska et al., 2014). Moreover, γS is present in the presynaptic axons of the hippocampus of PD and DLB patients but not found in healthy control group’s brains (Galvin et al., 1999). In DLB and in other NDDs such as AD, γS inclusions are found in the amygdala and the substantia nigra, interestingly, the aggregation of this protein is aided by the oxidation at the methionine in position 38, and colocalize with phosphorylated αS at position 129 (Surgucheva et al., 2014). Importantly, the oxidation of γS at M38, or Y39 (two of the easiest residues to oxidize in γS), create oligomers that can rapidly form oligomers and contribute to aggregation, and furthermore, seed aggregation of αS (Surgucheva et al., 2012). All these cases provide evidence to pursue a deeper analysis into the nature of γS and its potential for toxicity, investigating post-translation modified γS, which shows prion-like properties of seeding aggregation of the other synuclein. γS is also implicated in different cancers such as colon, pancreas, and breast contributing to progression of tumors acting through a variety of mechanisms (Pan et al., 2002; Inaba et al., 2005; Hua et al., 2009).
Emily Ong’s Thesis Inspired Substitution Mutants
Emily Ong in her senior thesis studied the familiar αS mutations, H50Q, G51D, and A53E, and the cause of their toxicity (See Appendix A). Different mutations can render a protein toxic. One way could be due to the loss of the original amino acid or the gain of the new one conferring pathology hallmarks of PD. Using both the budding and fission yeast model system, she created substitution mutants of these mutations in αS by switching the original amino acid with a new one from the four distinctive functional groups of amino acids. In the case of H50Q, she synthetized H50G (glycine, nonpolar), H50N (asparagine, polar), H50R (arginine, basic), and H50D (aspartic acid, acidic). Her results showed that the loss of the original histidine was conferring toxicity, presenting all substitutions toxic (Ong, Thesis, 2017). For the A53E, loss of the original amino acid was key to toxicity and was further supported by the presence of two other mutations in position 53 (A53T and A53V). On the other hand, G51D seemed to be toxic due to the gain of the aspartic acid, which showed similar toxicity to the substitution mutant G51E, also acidic (Ong, Thesis, 2017). This analysis inspired my first aim because she designed the substitution mutants to gain insights on what is driving toxicity. I learned how important the substitution idea was to understand better the mutants, and thus, I wanted to apply the same project design to the understudied βS mutants.
Ryan Osselborn’s Senior Project and My Contributions
Ryan Osselborn in his senior project he comparatively evaluated WT αS, βS, and γS in both low and high expression models using the budding yeast (See Appendix B). His senior project work started with contributions from myself in the tool making process. I helped Ryan and Tracey get all the necessary synuclein constructs and contributed data for their theses. From his work, he reported that αS and βS are differentially toxic, with the former being more, whereas γS is nontoxic in a high expression model. In a low expression model, no differences in toxicity were reported for any of the synuclein proteins. Even though no cytotoxicity is achieved with a low expression model, this system is still powerful too study what properties or altered cellular environments can enhance toxicity. Similarly, with a high expression system, we are able to study what elements decrease or provide a protection against toxicity.
In his senior project, Osselborn also reports βS the protein to have higher than expected molecular weight through western blot analysis. The change in size could be due to post translational modifications that the protein undergoes after being synthetized, specifically SUMOylation Nonetheless more information is needed to understand its size. In my thesis I aim to investigate what drives toxicity of βS and if I can make γS toxic. For this reason, all my senior thesis work is done in the high expression system.
Tracey Nassuna’s Thesis and My Contributions
Tracey Nassuna, in her senior thesis studied the molecular underlying of toxicity for the βS mutation P123H (See Appendix C). Using the budding yeast model system, Tracey Nassuna, and I, using the same strategy as Emily Ong, created substitution mutants for P123H βS following the same functional groups to investigate if the loss or the gain of the new amino acid is key to toxicity. We created in βS P123E (glutamic acid, acidic), P123R (arginine, basic), P123A (alanine, nonpolar), and P123N (asparagine, polar). For her thesis, I collected all the data with the high expression system. Nassuna and I reported that for P123H βS the gain of the new amino acid is the cause of the mutant toxicity since no other substitution mutant showed similar toxicity than the mutant. I am interested in continuing collecting data for the P123H mutant and expanding this work by also analyzing the second βS mutant V70M, by also creating substitution mutants with the same amino acids.
Furthermore, Tracey Nassuna in her thesis also studied the initial effects of swapping the known βS mutants (V70M and P123H) for αS. With my help, we created the swap mutant αS-V70M and αS-P128H, following sequence alignments and corresponding conserved sequences. She found that V70M αS showed similar toxicity that αS, whereas P128H αS showed less toxicity when compared to the former two, but similar to βS. I am interested in furthering this research by expanding on this work and creating swap mutants of known αS mutations into βS and γS, and βS mutations into γS.
Post translational modifications in synucleins
One way a protein can change function, structure, and localization after being synthetized is through post-translational modifications (PTM). PTM occur at specific sites where different chemical groups, such as methyl, are added to the protein. PTM are necessary for certain cellular processes such as ubiquitination, in which a ubiquitin molecule is added to a protein’s lysine residues and creates a signal for degradation in the proteasome (Ramazi & Zahiri, 2021). Another especially important type of PTM is methylation, attaching a methyl group to a protein producing an epigenetic regulator of gene expression (Ramazi & Zahiri, 2021). aS has many PTM sites linked to either facilitating or preventing formation of fibrils and membrane interactions (Ellis et al., 2001). PTM are classified as beneficial or harmful in the context of aS toxicity. Many studies in vivo and in vitro report that aS can get phosphorylated by kinases (CKI and CKII among others) at S87-P (Okochi et al., 2000), S125 -P (Ellis et al., 2001; Nakamura et al., 2001), and S129-P (Okochi et al., 2000; Anderson et al., 2006). Phosphorylation is considered a harmful event, increasing aS toxicity. Similarly, to αS, βS also gets phosphorylated at the C-terminus, S118-P (Hayashi, & Carver, 2022b). Based on sequences alignments the phosphorylation at position S118 is comparable to the one for aS at position S129 (Hayashi, & Carver, 2022b). No known serine residue is known to get phosphorylated in γS.
aS has high susceptibility to get nitrated, increasing oxygen and nitric stress, at the tyrosine residues in positions Y39, Y125, Y133, and Y136 (Sevcsik et al., 2011; Burai et al., 2015). Like phosphorylation, nitration is a harmful PTM. These tyrosine residues are critical in intermolecular interactions and cross-linking of the protein mediating oligomerization, which is not seen when these sites are blocked by replacing the tyrosine residue with a hydrophobic phenylalanine (Y39F, Y125F, Y133F, and Y136F; (Burai et al., 2015). Many of these tyrosine residues are conserved also in βS and γS, however, PTM in βS and γS are less studied and understood than those in αS.
Nonetheless, some PTM can be beneficial such as SUMOylation and acetylation. αS can get sumoylated at the SUMO accepting lysine residues sites, K96 and K102 (Krumova et al., 2011), and acetylated at K6 and K10 (de Oliveira et al., 2017). For SUMOylation, studies using mutants to block SUMO sites (K95R and K102R) showed that αS was more propense to aggregate and had higher cytotoxicity in the substantia nigra dopaminergic neurons (Hassanzadeh et al., 2023). Acetylation also decreases progression, propensity to aggregate, and the morphology of the aggregates, which had lower content of β-sheet content (Bell et al., 2022). Given the notable conservation of amino acid sequence between αS, βS, and γS, it follows that they share analogous PTM sites. The focus of my senior thesis pertains to the potential impact of the PTM described before in αS, in the context of βS and γS.
Gap in Knowledge
All literature in synucleinopathies and Lewy body diseases focuses on αS and its mutations, which are well characterized. Until recently, βS was believed to protect against αS toxicity, however, studies provided new links between βS and γS with NDDs. Two βS mutations, V70M and P123H, are causative agents in DLB. Nonetheless, the mechanisms behind toxicity are not well understood. γS also shows promising potential for being a causative candidate of toxicity, however, the nature of its potential to be toxic is unknown. Their toxicity potential and neurodegeneration mechanisms are worth evaluation more fully. I am eager to ask what drives the toxicity of the two b-Synuclein mutants: The loss of the original amino acid (V70, P123) or gain of the mutants (70M, 123H)? Secondly, six/four amino acids linked with the nine aS genetic mutations are conserved in βS and γS, respectively, as is βS V70 in both aS and γS, and βS P123 in aS (P128). I wonder about the effect of introducing the aS mutations into βS and γS, and βS mutations into aS and γS. Gaining this knowledge will illuminate the molecular determinants of the bS and γS’ elusive toxicity.
Furthermore, like aS, βS is modified post-translationally, however the effect of disrupted cellular environments (mitochondrial stress, nitrative stress, lysosomal dysfunction) and post-translational site mutations remain unassessed. Even less is known about γS post-translational modifications and these other disruptions. I am interested in exploring these in βS and γS for the known aS’ phosphorylation, SUMOylation, and glycation sites for those that are conserved in βS and γS. The gaps in knowledge of this thesis are represented in Figure 6.
Yeast as a Model Organism
Many labs around the world use different model organisms to study synucleinopathies and PD, such as Drosophila, C. elegans, rodents, neuronal cell cultures. All of these provide benefits and disadvantages to study synucleinopathies. Some of the most popular models such as rodents or neuronal cells which have easier translation into humans present many obstacles such as difficult to manipulate and difficult for high/low-throughput screening and genetic analysis. For the purpose of this thesis, the DebBurman lab and many others choose to use an unconventional and powerful model organism: Budding Yeast (Saccharomyces cerevisiae, S. cerevisiae).
- cerevisiae have a fast reproduction time, only taking between 1.5 to 3 hours to replicate being a single-celled organisms belonging to the fungus eukaryotes. Yeast has a small, sequenced genome (approximately 6,000 genes), with high similarities between humans and yeast sharing an overlapping 32% between amino acid sequences (Leslie, 2015). Many human genes linked with NDDs have yeast homologs, such as PARK2 that codes for the Parkin protein, which is the cytosolic ubiquitin-E3-ligase, involved in ubiquitination and where mutations of PARK2 lead to PD (Konovalova et al., 2015). Other yeast homologs and orthologs from human genes are SCA2 associated with spinocerebellar ataxia type 2, SOD1 in ALS, and ATP7B mutations leading to transporter dysfunction linked to Wilson Disease (Allendoerfer et al., 2008). Yeast also has a highly conserved stress response, protein folding, modification, and degradation pathways as humans (Allendoerfer et al, 2008). All these similarities allow for yeast to be used as a powerful and insightful model organism to study the neuropathology and protein interactions these diseases are characterized by. Almost half of the yeast genes have human counterparts, which make it easier to use yeast as a genetic tool. Yeast is amazingly easy to manipulate the yeast genome and introduce human genes without losing functionality, as well as having knock-out knock-in techniques (Allendoerfer et al, 2008). In the case of this thesis, we use aS, βS, and γS, but have no yeast homolog. Outeiro and Lindquist were the first to establish a PD yeast model in 2003 by reproducing the characteristics of aS toxicity in pathology seen in patients (Outeiro & Lindquist, 2003).
Since then, many labs use yeast for a PD model including the DebBurman lab, which used both the budding yeast and the fission yeast (Schizosaccharomyces pombe) to contribute to a larger community. The DebBurman lab uses a high copy 2-micron plasmid in which aS, βS, or γS genes can be inserted and expressed in the yeast. Many past members used this system to study the familial older and newer mutants such as Brandis et al., 2006; Fiske et al., 2011; Sharma et al, 2006; Emily Ong (Thesis, 2017), Amanda Grassel (Thesis, 2023), and Tracey Nassuna (Thesis, 2023).
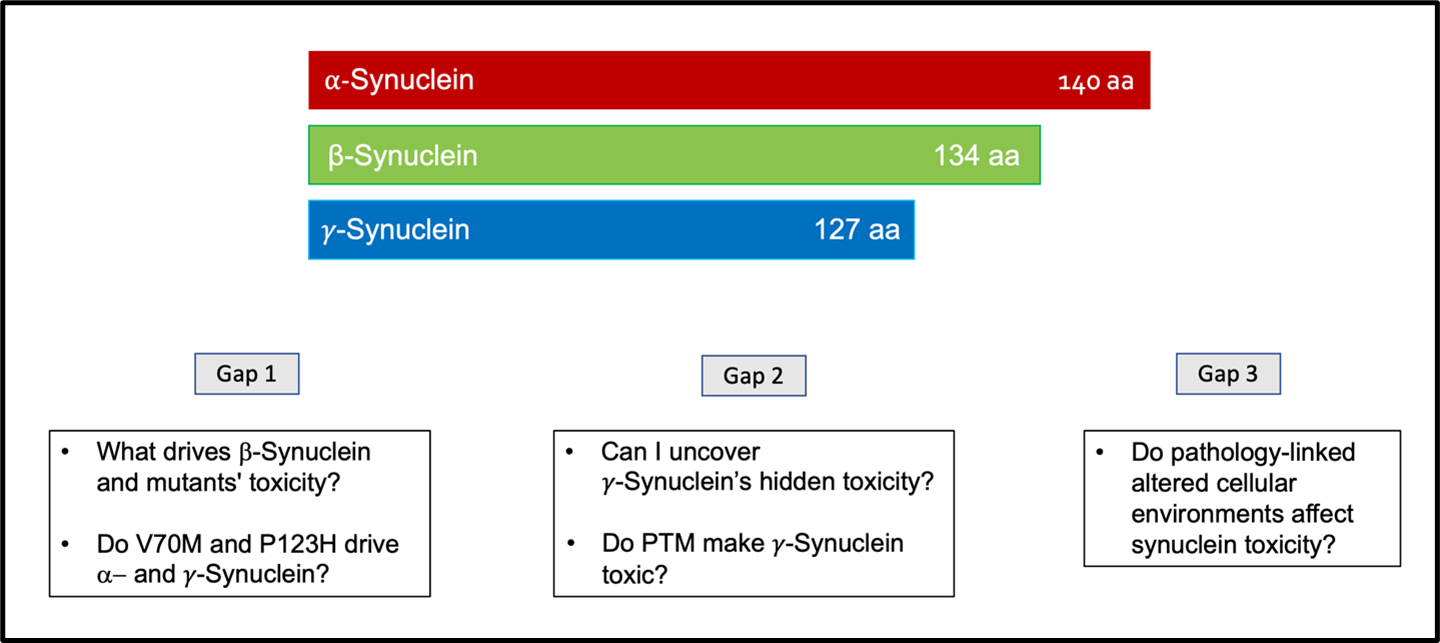
Figure 6. Gaps in knowledge that drive the investigation and comparison between the synuclein proteins. Gap 1 is to understand what drives the bS and the bS mutants’ toxicity. As well as understand how critical are the mutations V70M and P123H for toxicity by looking at them in aS and gS. Gap 2 is to investigate if I can uncover gS hidden toxicity, by showing toxicity, and study how post-translational modifications might affect gS. Gap 3 is to understand the effects of disease-linked altered cellular environments on the synucleins’ toxicity.
Hypotheses and Aims
I propose to add insight into three hypotheses:
1) Either the loss of the original amino acid or gain of the mutant is key to V70M and P123H βS toxicity.
2) At least some of the known aS and βS mutations when swapped onto each other (aS, βS, and γS) will increase their toxicities.
3) Finally, post-translational modifications and altered neurodegeneration-related environments will regulate βS and γS toxicity.
Chapter 1
The goal for chapter 1 was to create all the 30 mutants necessary for all aims in my thesis. For Aim 1, my goal was to create eight substitution mutations. For Aim 2 to create swap mutants. For Aim 3, to evaluate covalent modifications with PTM site mutants. For my project, I was able to successfully create 25 of the mutants necessary in budding yeast, as described in chapter 1 of results. All plasmids used in this thesis use a high expression system, having GFP in p426 vector.
Chapter 2
The goal of this chapter is to answer Aim 1 and Hypothesis 1 by characterizing the βS substitution mutants for both V70M and P123H. I am examining toxicity, localization, and expression of the synuclein as compared to WT βS using three assays: Serial dilution spotting, GFP fluorescent microscopy, and Western blot, in two control strains W303 and BY4741.
I predicted for V70M substitutions, the mutant that conserved the hydrophobicity properties of WT βS and the V70M mutant will show similar toxicity, while the other substitution mutants would have non-toxic properties. For P123H, I predicted all mutants would show toxicity because of proline loss. My findings support the first hypothesis, and I found position 70th is critical for toxicity and aggregation, while for P123H, the polar and basic residues matter.
Chapter 3
My goal for this chapter was to investigate if the presence of specific amino acids are crucial toxicity regulators. I created swap mutants by introducing the disease-linked mutations from aS and bS into each other and γS. For aS mutations into bS and γS, I predicted that bS toxicity would be aggravated by aS mutations, and γS will show potential for toxicity. I also predicted aS toxicity would also be aggravated by bS mutants. I was to show that aS swaps had differential toxicity, aS-V70M showed similar toxicity to WT aS, whereas aS-P128H to WT bS. Contrary to my hypothesis, γS swaps showed no toxicity or aggregation.
Chapter 4
My aim was to study covalent modifications of bS and γS by creating PTM site mutants. Based on previous literature of oxidization of γS, I predicted that the mutant that mimics nitration would make γS toxic, while those mutants that block nitration and mimic SUMOylation and acetylation will conserve the non-toxic properties of γS. As for bS, I predicted increased pathogenicity when nitration and phosphorylation are mimicked, but a protective phenotype when acetylation, SUMOylation are mimicked, but nitration and phosphorylation, are blocked. My findings are preliminary data that shows that toxicity in bS is not affected, and the PTM do not make γS toxic.
Chapter 5
For my last chapter, my goal is to continue answering aim 3 by studying bS and γS in yeast strains altered for different cellular environments. In particular, for altered levels of nitrative stress, decreased glycation, and increased acetylation. These strains will be compared to the control strain BY4741. I used cox5A∆ for elevated levels of nitrative stress and cox5B∆ for low levels of nitrative stress. Then, I used sirt2∆ for high levels of acetylation, and glo1∆ for decreased levels of glycation. This work was done with Leslie Casares ’26. Our findings show that high nitrative stress can aggregate bS and its mutants’ toxicity, and even make γS toxic. While decreasing glycation and increasing acetylation is protective to the synucleins.
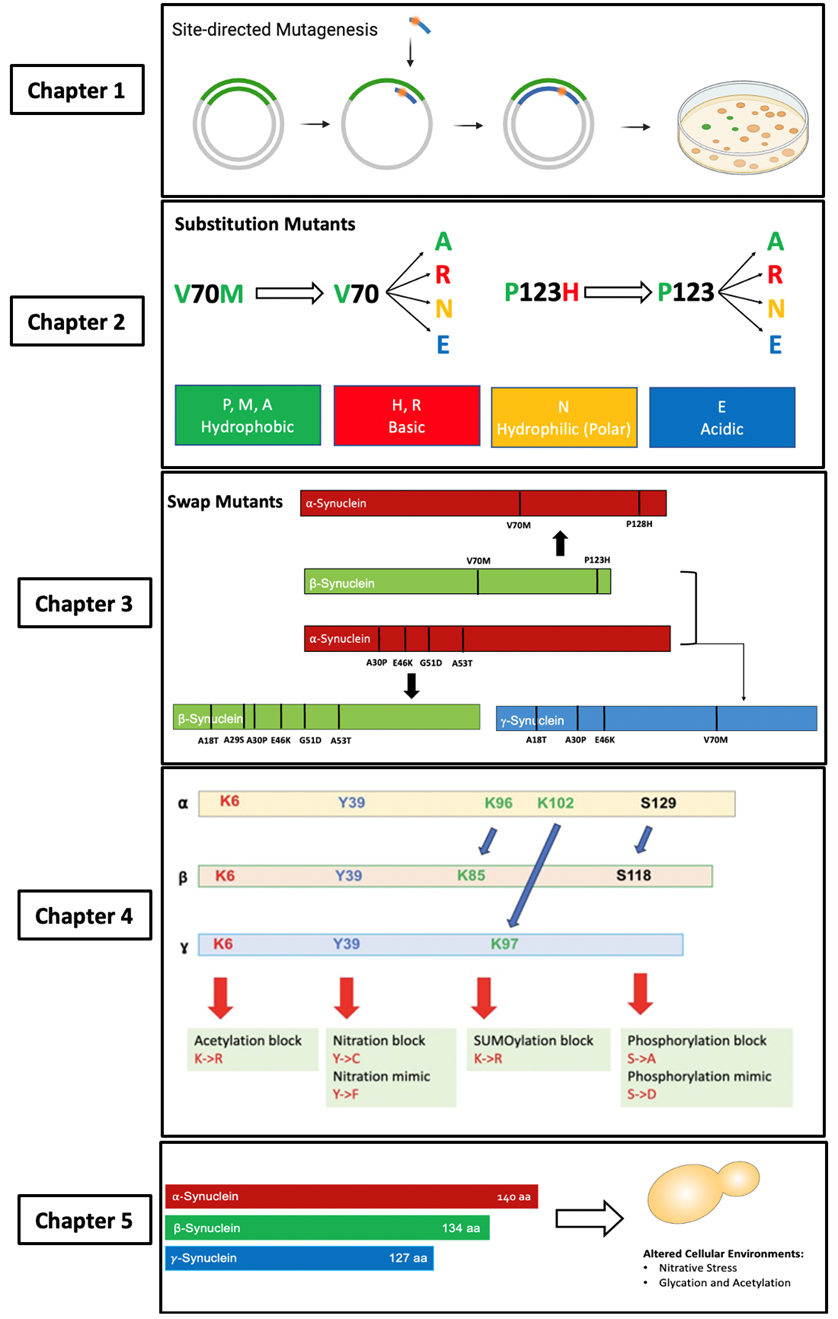
Figure 7. Project overview of each chapter. 1) Create the substitution, swap, and PTM site mutants by introducing the mutations into αS, βS, and γS, using a site-directed mutagenesis technique. 2) Examine if the loss of the original amino acid (V70 or P123) or the gain of a new amino acid (70M or 123H) is the determinant of the βS mutants’ toxicity. 3) Examine the impact of swap mutants of αS, βS, and γS to investigate if these would aggravate αS, βS, and make γS toxic. 4) Characterize and study if PTM site mutants for βS and γS show similar toxicity properties than αS. 5) Use different yeast strain modelling altered neurodegeneration-related cellular environments and their effects on βS and γS ‘s potential toxicity.
Note: Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College. Articles published within Eukaryon should not be cited in bibliographies. Material contained herein should be treated as personal communication and should be cited as such only with the consent of the author.