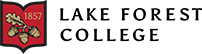PMA Activates Non-Amyloidogenic APP Processing While BACE1 Overexpression Drives Amyloidogenesis in the CHO 695 Alzheimer’s Disease Model
*This author wrote this paper for Neuroscience 387: Investigation of Neurodegenration taught by Dr. Blaine D. Moore.
Abstract
Alzheimer’s disease is a relentless neurological disorder that progressively impairs cognitive function, primarily in older individuals. Despite existing treatments to alleviate disease symptoms, there is no definitive therapy to halt or reverse disease progression. The aggregation of amyloid-β proteins into extracellular plaques remains a hallmark of the disease. Current approaches aim to alter the competitive proteolysis of APP, selecting the non-amyloidogenic pathway to reduce the formation of Aβ peptides. This work set out to modulate the competitive cleavage of APP in CHO695 cells expressing human isoform-695 of APP, demonstrating the competitive nature of the pathways. The CHO695 cells were treated with PMA to drive α-secretase activity, tipping the competitive cleavage in favor of the non-amyloidogenic pathway. The cells secreted Aβ into media, which was then analyzed by ELISA to quantify total secreted Aβ. The present study’s hypothesis follows that α-secretase stimulation with PMA will yield decreased Aβ secretion in comparison to the β-secretase transfection condition. Our results displayed a shift towards the non-amyloidogenic pathway for the stimulation of α-secretase with PMA. The GFP:BACE1 transfection resulted in increased Aβ secretion. These findings support the hypothesis that stimulation of α-secretase through the PMA-PKC pathway produces significantly less secreted Aβ in contrast to the overexpression of β-secretase. The CHO695 model system used may not represent the full complexity of APP processing that occurs in neurons. Additionally, the activation of PKC with PMA can result in the activation of differing PKC isoforms which exhibit functions beyond APP processing. Future studies should seek to employ more selective inhibitors of β-secretase, to spare its separate essential functions, and commission work in neuronal or in vivo systems. Convincingly, therapies that accurately modulate APP processing to minimize Aβ production, while mitigating unintended side effects, have significant potential in directing AD progression.
Introduction
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases in the world, affecting over 50 million individuals worldwide. AD is characterized by progressive cognitive decline and severe impairment of memory, cognitive function, and reasoning abilities. The disease’s pathology includes two key hallmarks, extracellular amyloid β (Aβ) plaques and intracellular neurofibrillary tau tangles. Amyloid β plaques result from the aggregation of Aβ proteins, while tangles result from hyperphosphorylated tau proteins which disrupt normal neuronal function (Barage & Sonawane, 2015). Both disease hallmarks disrupt synaptic function and neuronal integrity, eventually leading to widespread brain deterioration. Although tau tangles contribute to disease progression, the accumulation of Aβ peptides into plaques is seen as the initiating event of the disease, known as the amyloid cascade hypothesis (Barage & Sonawane, 2015).
The amyloid cascade hypothesis suggests that Aβ aggregation resulting from amyloid precursor protein (APP) processing is central to AD pathogenesis. APP is a transmembrane protein found predominantly in neuronal cells and can undergo proteolytic cleavage via two pathways: the amyloidogenic pathway which generates Aβ proteins, and the non-amyloidogenic pathway which precludes Aβ protein formation (Cai et al., 2001). In the amyloidogenic pathway, β-secretase, or BACE1, cleaves APP to from the C99 fragment which is further proteolyzed by γ-secretase to release the Aβ protein. These proteins exist in two major isoforms, Aβ40 and Aβ42 (Barage & Sonawane, 2015). The latter are highly prone to aggregation, forming oligomers and eventually toxic Aβ plaques. On the other hand, the non-amyloidogenic pathway involves α-secretase, or TACE, which cleaves APP within the Aβ sequence, thus preventing its formation. This pathway also generates the sAPPα fragment, which has roles in both neuroprotection and synaptic plasticity (Barage & Sonawane, 2015; Hung et al., 1993). The competition between these APP processing pathways influences neuronal health, with the amyloidogenic pathway leading to pathological changes associated with AD.
A key component in the non-amyloidogenic pathway of APP processing is TACE. TACE is the primary α-secretase that cleaves APP at the α-site to produce the sAPPα fragment and reduce Aβ protein production. Activation of protein kinase C (PKC) can be achieved through the use of phorbol esters such as phorbol 12-myristate 13-acetate or PMA. PMA leads to TACE phosphorylation, causing upregulation in α-secretase activity and shifting APP processing toward the non-amyloidogenic pathway (Hung et al., 1993). Targeting TACE in this way is just one therapeutic potential that can be used to mitigate Aβ production. However, α-secretase must compete with β-secretase for APP as a substrate. Numerous factors can influence this competition, including but not limited to, enzyme location, substrate availability, and post-translation modifications, shifting the balance toward either amyloidogenic or non-amyloidogenic processing of APP (Skovronsky et al., 2000).
In the work we are doing in lab, we aim to further investigate the effects of regulating both the amyloidogenic and non-amyloidogenic pathways of Aβ production. To do this, we will employ PMA treatment to stimulate α-secretase activity as well as BACE1 transfection to enhance β-secretase cleavage in CHO695 cells, Chinese Hamster Ovarian cells expressing the 695 isoform of APP. Through these experiments, we will examine the impact of the two treatments on Aβ secretion levels, using ELISA to quantify secreted Aβ proteins and comparing results across conditions to test our hypothesis that increased α-secretase activity will reduce Aβ levels relative to β-secretase enhanced conditions.
Methods
Passaging CHO695 Cells in T-25 Flasks
CHO695 cells were cultured in T-25 flasks and maintained in a pinkish, clear medium. Every two days, or when medium turned orange, cells were passaged into new T-25 flasks. If contamination was present in the culture, indicated by frothy or cloudy medium, the cells weren’t passaged. To passage the cells, the surface of the hood was sterilized using 70% ethanol and all required materials, versine, trypsin, and complete medium were placed into the hood. The old medium from the flask was drawn off into a liquid waste beaker and 2 mL of versine was added to the flask, carefully placing it in the bottom corner opposite the cells to avoid splashing. The flask was then rocked back and forth to ensure uniform coverage without liquid entering the neck. The versine was removed, and 0.5 mL of trypsin was added to detach cells. The flask was rocked back and forth and allowed to incubate at room temperature for two minutes. While cells were incubating, a new T-25 flask was prepared and labeled with relevant details (cell line, date, passage number). 5 mL of fresh complete medium were added to the new flask. After the trypsin was done incubating, the cells were detached by tapping the side of the flask with the palm to loosen the cells. The flask was placed under a microscope to confirm that the cells had detached from the bottom of the flask. The bottom of the flask was then rinsed with 7.5mL of feed medium. 1 mL (for a 1:8 split) or 2 mL (for a 1:4 split) of the cells were transferred into the new flask. The flask was rocked back and forth for uniform coverage, capped loosely, and placed in the incubator at 37°C.
Plating CHO695 Cells in 6-Well Plates
When the cells reached confluence, 48 hours after passaging, they were plated into a 6-well plate. The procedure is identical to passing cells into a new T-25 flask, except for the last few steps. After cells have been transferred into the new flask, the leftover cells are used for plating. The leftover cells are evenly suspended, and 4 mL of the leftover cells are moved into a 15 mL conical tube. 8.5 mL of fresh feed media are added to the tube and the contents of the tube are mixed gently. 2 mL of this mixture is then pipetted into each well of the 6-well plate and the plate is placed into the incubator at 37°C
PMA Treatment of CHO695 Cells
Cells that were previously plated and had reached confluence were used for PMA treatment. 3.5 mL of DMEM cell culture media was transferred to two 15 mL conical tubes, one labeled “+PMA” and one labeled “-PMA”. To the tube labeled “+PMA”, 3.5 uL of PMA was added and the contents of the tube were mixed gently. The 2 mL of cell culture media from each well was removed and placed in liquid waste. 1 mL of the “-PMA” media was pipetted into each of the top three wells of the plate, being careful not to dislodge the cells. 1 mL of the “+PMA” media was pipetted into each of the bottom three wells of the plate, being careful not to dislodge the cells. The lid of the plate was labeled with what treatment was in each well and the plate was placed in the incubator at 37°C for 2 hours.
BACE1 Transfection in CHO695 Cells
The day before transfection, cells were plated into a 6-well plate. On the day of transfection, a microscope was used to visually inspect the cells and make sure the cells are at appropriate confluence. In the hood, six sets of two microfuge tubes are filled with 500 uL of serum-free transfection media. Six of the tubes are labeled GP for GenePORTER, three tubes are labeled GFP 1-3, and the last three tubes are labeled GFP:BACE1 1-3. In the GP tubes, 15 uL of GenePORTER were added, in the GFP 1-3 tubes, 20 uL of GFP DNA stock were added, and in the GFP:BACE1 1-3 tubes, 20 uL of GFP:BACE1 DNA stock were added. The contents of one tube of GP were pipetted into one tube that contained DNA. This was done for the remaining five sets of tubes. The tubes were then allowed to incubate at room temperature for 20 minutes. After incubation, the plate of cells was obtained from the incubator, and in the hood, the culture medium was drawn off from every well. The DNA-GP mixtures were added to each well, with GFP 1-3 going in the top three wells and GFP:BACE1 1-3 going in the bottom three wells. The lid of the plate was labeled with what DNA mixture is in what well. The plate was then placed in the incubator at 37°C for 5 hours. After 5 hours, 1 mL of media containing 20% FBS was added to each well to stop the transfection. The plate was placed back into the incubator at 37°C and 5-10% CO2 and allowed to incubate overnight.
Harvesting Conditioned Media
To collect condition media, 1 mL of media from each well in a 6-well plate was transferred to an appropriately labeled tube. 2 uL of PMSF and 5 uL of PI are then pipetted into each of the tubes containing conditioned media, making sure to use a new pipette tip for each inhibitor. The tubes were inverted a few times to mix and placed on ice. After all conditioned media tubes were ready, they were centrifuged at 14k rpm for 20 minutes at 4°C to remove dead cells and any cellular debris. While the samples were spinning, new tubes were labeled to collect the supernatants. After the samples were done spinning, the supernatant from each tube was transferred into its corresponding supernatant tube. The supernatant tubes were stored in the -20°C freezer until they were needed for further analysis.
Harvesting Cell Lysates
From the same plate that the conditioned media samples were taken from, the remaining 1 mL of media in each well was removed and placed in liquid waste. 1 mL of 1x PBS was immediately added to each well, being careful not to dislodge the cells. After all the wells had PBS, the plate was gently swirled to wash the cells of any remaining media. The PBS was then removed from the first well and 750 uL of lysis buffer was added. A plastic scraper was used to gently scrape the bottom of the well and the resulting cell lysate was transferred into an appropriately labeled tube and placed on ice. This was repeated for the remaining five wells, making sure to wipe the scraper with a KimWipe and change pipette tips for every well. After all of the cell lysate samples were collected, they were sonicated to help break open any remaining cells. After sonication, all cell lysate samples were centrifuged at 14k rpm for 20 minutes at 4°C to remove dead cells and any cellular debris. While the samples were spinning, new tubes were labeled to collect the supernatants. After the samples were done spinning, the supernatant from each tube was transferred into its corresponding supernatant tube. The supernatant tubes were stored in the -20°C freezer until they were needed for further analysis.
ELISA to determine total secreted Aβ
To measure the total secreted Aβ secreted from the conditioned media samples, a human-specific Aβ sandwich kit was utilized to trap the Aβ in an “antibody sandwich”. The conditioned media samples were thawed and 50 µl was loaded to each respective well in a 96-well strip containing wells coated with Aβ 1-40 capture antibody. Then, 50 µL of Hu Aβ40 Detection Antibody solution was added to each well. The Hu Aβ40 Detection Antibody is used to coat the Aβ. Following this, the plate was set to shake on a shaking plate for 1.5 hours at room temperature. After the 1.5-hour incubation, the solution in the sample wells was drawn off and discarded to aspirate the cells, and the cells were washed 4 times with 300 µL of 1x wash buffer. Following the fourth wash, 100 µL of the secondary antibody solution was added to each well. The second antibody, HRP-anti-rabbit reporter-enzyme-linked antibody is raised against the Hu Aβ40 Detection Antibody. The plate was then placed on a shaking incubator at room temperature for 30 minutes. Then, the wells were aspirated and washed an additional four times with wash buffer, except for the fourth wash. The fourth wash was aspirated and chromogen and stop solution were added to the wells. The plate was then analyzed for absorbance of 450 nm of light to determine the total secreted Aβ.
BCA Analysis of Protein Concentration
To determine how much protein was in the cell lysates, BCA technique was utilized. Bovine serum albumin (BSA) was purchased at a known concentration of 2mg/mL and serially diluted through a two-fold dilution to create several final concentrations of 2.0, 1.0, 0.5, 0.25, 0.125, 0.0625, and 0.03125 mg/mL. Several microcentrifuge tubes were labeled with the concentrations above and 50 µL of lysis buffer was added to each microcentrifuge tube with the exception of the 2.0 mg/mL tube which received 100 µL of BSA. 50 µL was transferred from the 2.0 mg/mL tube to the 1.0 mg/mL tube using a p200 pipette and the 1.0 mg/mL tube was vortexed well. This vortex/transfer process was repeated for the remaining concentration tubes until all dilutions were complete. The tubes were then transferred to a 96-well plate that was divided into three zones with each zone done in duplicate. Zone 1 was the blank baseline and each well received 10 µL of lysis buffer. Zone 2 had 10 µL of the several final concentrations of BSA individually pipetted into a respective well. Zone 3 was set up so each well received 10 µL of the 6 unique lysate samples. To prepare the BCA working solution, component B of the reagent 4% cupric sulfate was diluted from a 50x concentration to a 1x concentration with component A of the reagent sodium carbonate, sodium bicarbonate, bicinchoninic acid (BCA), sodium tartrate in 0.1 M sodium hydroxide. To achieve a final volume of 7.0 mL working solution, 140 µL of B was diluted in 6.86 µL of A. 200 µL of working solution was then pipetted into each of the 22 wells before the 96 well plate was set to incubate at 37°C for about 10 minutes. After about 10 minutes, the 96 well plate was analyzed for absorbance by a microplate reader at wavelength of light 562 nm. This analysis returned the protein sample concentrations as well as a standard curve.
Microscopy
Bright-field microscopy was performed with a DMX1200 Nikon inverted microscope to monitor and image the cells in a confluent monolayer. During the transfections of the CHO695 cells, epifluorescence was performed to verify the expression of cDNA using a DXM1200 Nikon inverted microscope and ACT-1 software.
Statistics
A two-tailed, two-sample t-test with unequal variance was performed to determine if there was a statistically significant difference between the (+) PMA and (-) PMA protein levels.
Results
Introduction
To observe the effects of α-secretase stimulation and β-secretase overexpression on Aβ processing, each pathway was selectively targeted. CHO695 cells were treated with PMA in triplicate to drive α secretase activity and in another experiment, transfected with β-secretase to enhance β secretase levels. Following the treatments, the total secreted Aβ levels were quantified by an ELISA assay and normalized to cell lysates via BCA assay to compare with controls.
PMA Treatment Promotes the Non-Amyloidogenic Pathway in APP Processing
Bright-field microscopy of untreated CHO695 cells was used to confirm the presence of a confluent monolayer of cells. The CHO695 cells displayed their typical shape and structure and tight adhesion to one another (Figure 1A). These characteristics indicate healthy, well-maintained cell cultures at baseline. The cells were plated in a six-well plate and three of the wells didn’t receive PMA treatment and the other three did. After a two-hour treatment with PMA, bright-field microscopy revealed no significant changes in the cell shape and structure or the integrity of the monolayer (Figure 1B).
To evaluate the impact of PMA treatment on the secretion of Aβ, conditioned media from the wells of both treated and untreated cells were analyzed using ELISA. After normalization of the ELISA data to cell lysates via BCA assay (data not shown), the relative Aβ concentration for the -PMA samples were 1485.3, 1387.5, and 1410.1 and the relative Aβ concentration for the +PMA samples were 306.8, 299.2, and 338.9. Quantitative analysis revealed a statistically significant reduction, around 78%, in the levels of Aβ found in the conditioned media of PMA-treated cells compared to untreated controls (p = 0.0001, Figure 3). The reduction in Aβ secretion was consistent across biological replicates, indicating the reproducibility of activating the α secretase pathway. The ELISA measurements were normalized to total protein concentrations, determined with cell lysates via BCA assay, to control for potential differences in cell density between experimental groups.
The observed reduction in Aβ secretion supports our hypothesis that PMA treatment influences APP processing. By maintaining cell viability and structure while reducing the levels of secreted Aβ, these results suggest that PMA causes a shift in the APP processing equilibrium favoring reduced amyloidogenic activity.

Figure 1. Bright-field microscopy images of both untreated and post-PMA treatment CHO695 confluent cell monolayers covering a culture well. (a) The cells making up the monolayer are spindle-shaped, with minimal spacing between adjacent cells. The nuclei are round and visible, indicative of a healthy, proliferative monolayer. Magnification set to 250x. (b) The cell monolayer was treated with 3.5 mL of DMEM medium and 3.5 μL of PMA. The image was captured 2 hours after PMA treatment. No significant changes were observed in the monolayer. Magnification set to 250x.
BACE1 Transfection Enhances β-Secretase Activity
To assess the effects of overexpression of β-secretase on APP processing, CHO695 cells were transfected with GFP:BACE1 cDNA. The cells were plated in a six-well plate and three of the wells received only GFP cDNA while the other three received GFP:BACE1 cDNA. Epifluorescence microscopy was used to verify successful transfection, which revealed robust GFP fluorescence in transfected cells (Figure 2A). The fluorescence signal suggests that the cDNA was predominantly located in intracellular compartments like the Golgi apparatus and endosomes where BACE1 is known to be active (Figure 2B). This aligns with the functional role of BACE1 in APP cleavage, as β-secretase activity is primarily seen in organelles like the Golgi apparatus and endosomes.
To quantify the effect of BACE1 overexpression on Aβ production, conditioned media from transfected and non-transfected cells were analyzed using ELISA. After normalization of the ELISA data to cell lysates via BCA assay, the relative Aβ concentrations for the GFP samples were 909.9, 1107.3, and 966.2 and the relative Aβ concentrations for the GFP:BACE1 samples were 1555.9, 1695.3, and 1910.7. Transfected cells showed a statistically significant increase in Aβ secretion, around 73%, compared to non-transfected controls (p = 0.007, Figure 4). This increase was consistent across experimental replicates. ELISA results were normalized to total protein concentrations in cell lysates, measured via BCA assay, to account for variations in transfection efficiency, cell density, or overall protein output. This normalization made sure that the differences in Aβ levels between experimental groups reflected changes in APP processing specifically attributable to BACE1 overexpression.
The data showed that cells overexpressing GFP:BACE1 produced substantially higher levels of Aβ compared to non-transfected controls. This increase in Aβ secretion provides direct evidence of the role of BACE1 in driving the β secretase pathway in APP processing. Bright-field microscopy of the transfected cells also showed no structural abnormalities in the cells, such as irregular shape or detachment, suggesting that the observed changes in Aβ secretion were specifically due to the transfection. Together, these results confirm that overexpression of BACE1 significantly enhances amyloidogenic cleavage of APP, leading to increased Aβ secretion under experimental conditions.
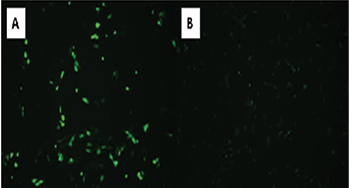
Figure 2. Epifluorescence microscopy of CHO695 cells transfected with GFP and GFP:BACE1 cDNA. (a) CHO 695 cells transfected with GFP cDNA displayed robust fluorescence, verifying transfection with the plasmid containing the GFP gene. (b) Fluorescence of cells transfected with GFP:BACE1 cDNA was visible around intracellular compartments, suggesting the localization of BACE1 to its typical active sites: the Golgi and endosomes.
Comparative Analysis
Normalized ELISA data from the PMA treatment and GFP:BACE1 transfection were compared to evaluate the opposing effects of α-secretase activation and β-secretase overexpression on levels of Aβ secretion (Figures 3 and 4). The data revealed an inverse relationship between PMA treatment and GFP:BACE1 transfection. Cells treated with PMA exhibited the lowest levels of Aβ secretion, with normalized values significantly reduced compared to untreated controls (p = 0.0001). In contrast, GFP:BACE1 transfected cells demonstrated the highest levels of Aβ secretion, with values significantly elevated relative to both untreated controls and PMA-treated cells (p = 0.007). The untreated control group in both the PMA treatment and GFP:BACE1 transfection showed intermediate levels of Aβ secretion, providing a baseline for comparison, and highlighting the balance between α and β secretase in APP processing under normal conditions.
The PMA-treated group consistently showed the lowest Aβ secretion across all replicates, with normalized ELISA values reflecting a substantial reduction of the β secretase pathway. This result was reproducible across independent experiments, indicating that PMA effectively shifts APP cleavage towards the α-secretase pathway, which precludes the formation of Aβ peptides. Statistical analysis confirmed that the reduction in Aβ secretion in PMA-treated cells was highly significant compared to untreated controls (p = 0.0001), demonstrating the impact of α-secretase activation in shifting the equilibrium in APP processing.
On the other hand, the GFP:BACE1 transfected group showed a stark increase in Aβ secretion, with normalized ELISA values significantly exceeding those of both untreated and PMA-treated cells (p = 0.007). The consistent elevation in Aβ levels across multiple replicates highlights the effect of BACE1 overexpression in driving the β secretase pathway. The normalized ELISA data ensured that these findings were independent of variations in cell density or overall protein levels, showing that the observed increase in Aβ secretion directly resulted from enhanced β secretase activity.
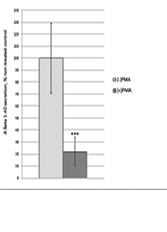 Figure 3. Bar graph displaying normalized A Β 1-40 secretion levels as % of non-treated control for PMA treated cells. The PMA treatment condition cells represented on the right (dark grey), displayed a statistically significant (77.94%/p = 0.0001) decrease in A Β level in comparison to the non-treated control. The non-treated control received no PMA treatment. The A Β levels were recorded using normalized ELISA data.
Figure 3. Bar graph displaying normalized A Β 1-40 secretion levels as % of non-treated control for PMA treated cells. The PMA treatment condition cells represented on the right (dark grey), displayed a statistically significant (77.94%/p = 0.0001) decrease in A Β level in comparison to the non-treated control. The non-treated control received no PMA treatment. The A Β levels were recorded using normalized ELISA data.

Figure 4. Bar graph displaying normalized A Β 1-40 secretion levels as % of non-treated control for GFP:BACE1 transfected cells. The BACE transfection treatment condition represented on the right (dark grey), displayed a statistically significant (73.02%/ p = 0.007) increase in A Β level in comparison to the non-treated control (GFP). The non-treated control was transfected only with GFP and not BACE. The A Β levels were recorded using normalized ELISA data of the replicates.
Discussion
Introduction
To interpret the effects of modulating α-secretase and β-secretase activity on Aβ processing, we investigated how each pathway contributes to APP processing and Aβ secretion. The treatments, PMA-induced activation of α-secretase and BACE1 overexpression to enhance β-secretase activity, provided a way to explore the balance between the non-amyloidogenic and amyloidogenic pathways. These experiments also help us gain a deeper understanding of the mechanisms that drive Aβ production and potential therapeutic options for mitigating Aβ plaque formation.
PMA Treatment Promotes the Non-Amyloidogenic Pathway in APP Processing
Our findings provide evidence that PMA treatment significantly reduces Aβ secretion in CHO695 cells. This reduction in Aβ levels is likely due to PMA-induced activation of protein kinase C (PKC), which has been shown to enhance α-secretase activity (Hung et al., 1993; Skovronsky et al., 2000). PKC activation promotes a shift in the cleavage of amyloid precursor protein (APP) from the amyloidogenic β secretase pathway to the non-amyloidogenic α secretase pathway. Specifically, α secretase cleaves APP within the Aβ sequence, producing sAPPα which does not produce Aβ peptides. In contrast, cleavage by β secretase (BACE1) produces the amyloidogenic Aβ peptides, which are used in the formation of plaques characteristic of Alzheimer’s disease.
The significant reduction in Aβ secretion in PMA-treated cells, as shown by the ELISA data, aligns with previous studies suggesting that PKC activation via phorbol esters like PMA increases α secretase activity, thus promoting non-amyloidogenic APP processing (Hung et al., 1993; Skovronsky et al., 2000). Our data further confirm this mechanism in CHO695 cells, showing that PMA treatment effectively reduces the generation of Aβ peptides by shifting the balance between these two competing pathways.
The lack of observed structural changes in PMA-treated cells, as seen by bright-field microscopy (Figure 1), supports the conclusion that the reduction in Aβ secretion is likely due to a shift in APP processing rather than any changes in cell function. The absence of any disruptions to cell structure, such as rounding or detachment, suggest that PMA treatment does not induce cellular dysfunction. This is a significant observation, as it implies that the activation of PKC can alter the processing of APP without disrupting other cellular processes, allowing for more targeted therapeutics.
These findings also underscore the potential for PKC activation to serve as a possible therapeutic option for Alzheimer’s disease. By promoting the α secretase pathway, PKC activators like PMA may reduce the production of Aβ peptides and, consequently, the formation of amyloid plaques. This could offer a more specific mechanism of action compared to traditional approaches that target β secretase or gamma-secretase, which are involved in cleaving other important substrates beyond APP. For example, inhibiting β secretase can have unintended effects on other cellular processes, including memory and cognition, due to the essential role of BACE1 in synaptic plasticity. In contrast, PKC activation may offer a more selective approach, reducing Aβ levels while avoiding major disruption to other cellular pathways. Past studies have looked at the therapeutic effects of PKC activators, specifically bryostatin-1 due it already being used in clinical studies for cancer and due to PMA’s tumor-promoting activity. By treating Alzheimer’s disease transgenic mice with bryostatin-1, researchers demonstrated that it was able to activate PKC and lead to a lower level of Aβ40 and Aβ42 in the brain of transgenic mice, while remaining low in toxicity (Etcheberrigaray et al., 2004).
Furthermore, the observed shift towards the α secretase pathway in response to PMA treatment suggests a competitive relationship between the α and β secretase pathways. This competition for APP substrate has important implications for therapeutic strategies aimed at reducing Aβ levels. Enhancing the α secretase pathway or inhibiting β secretase activity could be a possible avenue for treatment. Although there are currently no studies that explore the dose-response of PMA treatment on APP processing, there are studies that investigate the response of bryostatin-1 to identify the optimal concentration and duration that shifts APP processing toward the α secretase pathway without interfering with other cellular functions (Yi et al., 2012).
While our data shows the efficacy of PMA in reducing levels of Aβ secretion in CHO695 cells, future studies are needed to determine the relevance of these findings in more physiologically relevant systems, such as neuronal cultures or animal models of Alzheimer’s disease. Neurons, which naturally express different PKC isoforms and possess distinct APP processing dynamics, may respond differently to PKC activation than CHO695 cells. Additionally, the impact of PKC activation on other signaling pathways in neurons, including those related to synaptic plasticity and memory, should be further explored to ensure that long-term PKC activation does not lead to unintended effects on neuronal health and function.
In summary, our results suggest that PMA-induced PKC activation promotes a shift towards non-amyloidogenic APP processing, leading to a reduction in Aβ secretion. This mechanism of action could serve as a promising strategy for reducing amyloid plaque formation in Alzheimer’s disease. However, further studies in neuronal models and in vivo systems are necessary to confirm the therapeutic potential of PKC activation and evaluate its long-term effects.
BACE1 Transfection Enhances Β-Secretase Activity
In contrast to the effects of PMA treatment, our findings show that BACE1 overexpression through GFP:BACE1 transfection results in a significant increase in levels of Aβ secretion. This outcome is consistent with previous studies that have established BACE1 as the primary β secretase responsible for the amyloidogenic cleavage of APP (Cai et al., 2001). Β-secretase activity leads to the formation of Aβ peptides by cleaving APP at the β-site, which generates the sAPPβ fragment and the C99 fragment. The C99 fragment is then further processed by γ-secretase through sequential proteolysis to release various forms of Aβ, including Aβ40 and the toxic Aβ42 species that aggregate to form amyloid plaques, a key feature of Alzheimer’s disease pathology.
Our ELISA data shows that the elevation in Aβ secretion following GFP:BACE1 transfection directly correlates with enhanced β-secretase activity. The increase in Aβ levels serves as a marker of the role of BACE1 in promoting amyloidogenic APP processing. This not only supports the model of APP cleavage by BACE1 but also underscores the potential therapeutic strategy of targeting BACE1 to reduce Aβ accumulation in Alzheimer’s disease. However, these results also emphasize the challenge of inhibiting BACE1 without disrupting its essential physiological functions, such as its role in synaptic plasticity and neuronal signaling.
The fluorescence microscopy results provide insights into the intracellular localization of BACE1. The GFP:BACE1 cDNA was predominantly localized in compartments like the Golgi apparatus and endosomes, which are critical sites for APP cleavage. The Golgi apparatus is a primary site where APP is sorted and processed, with BACE1 being concentrated in these regions to cleave APP before it reaches the cell surface. The specific localization of GFP:BACE1 cDNA to these intracellular compartments reinforces the idea that BACE1’s function in APP processing is tightly regulated by its localization.
This finding also has important implications for therapeutic targeting of BACE1. Since β secretase activity is concentrated in the Golgi apparatus and endosomes, strategies aimed at modulating BACE1 activity could focus on these locations to specifically inhibit APP cleavage without affecting other cellular processes. For example, small molecules or peptides that selectively bind to BACE1 in the Golgi or endosomes could be developed as inhibitors, potentially minimizing effects on other BACE1 substrates.
The increase in Aβ secretion levels following BACE1 transfection also highlights the competitive nature of APP processing between the amyloidogenic and non-amyloidogenic pathways. The transfection of GFP:BACE1 not only elevated β secretase activity but also reduced the availability of APP for cleavage by α secretase, which operates through a competing pathway. This suggests that by increasing the activity of β secretase, APP processing is skewed toward the amyloidogenic route, leading to increased Aβ production. This competitive balance between α and β secretase pathways is a key factor in modulating Aβ levels and understanding how to manipulate this balance therapeutically could have significant implications for Alzheimer’s disease treatment.
The overexpression of BACE1 can also be used as a useful model for studying pathology in Alzheimer’s disease. The overproduction of the Aβ peptide, especially Aβ42, is believed to play a crucial role in the formation of amyloid plaques, which disrupt neuronal function and contribute to cognitive decline. By enhancing β secretase activity, we can simulate the pathological conditions seen in Alzheimer’s patients, providing a valuable model for testing potential drug and therapies aimed at reducing Aβ levels or inhibiting its aggregation.
In conclusion, the overexpression of BACE1 via GFP:BACE1 transfection provides strong evidence for the role of β secretase in amyloidogenic APP processing and Aβ production. Our results confirm the role of BACE1 in generating Aβ and also highlight the potential for therapeutic strategies aimed at modulating β secretase activity. However, the challenge remains in selectively inhibiting BACE1 to reduce Aβ production without disrupting its essential physiological functions, and future research will be crucial in developing more specific and effective therapeutic strategies for Alzheimer’s disease.
Comparative Analysis
The comparison of normalized Aβ levels across these different conditions underscores a competitive dynamic between α and β secretase pathways in APP processing. PMA-treated cells, with activated α-secretase, exhibited a significant reduction in Aβ secretion, suggesting that α secretase processing effectively reduces the substrate available for β secretase cleavage. In contrast, GFP:BACE1 transfected cells showed the opposite trend, with elevated β secretase activity driving amyloidogenic processing even in the presence of endogenous α-secretase activity.
The untreated control group’s intermediate Aβ levels reflect the balance of endogenous α and β secretase activities under baseline conditions. This balance provides a point of reference, demonstrating how experimental manipulation of the cells, such as PMA treatment and GFP:BACE1 transfection shifts APP cleavage dynamics. The significant differences in Aβ secretion between PMA-treated and GFP:BACE1 transfected cells highlight the opposing effects of these manipulations and the potential for modulating APP processing.
Implications for Alzheimer’s Disease Pathology
The findings from this study have significant implications for our understanding of Alzheimer’s disease pathology, particularly in terms of how APP processing influences Aβ production and the development of amyloid plaques, a hallmark of the disease. The reduction in Aβ secretion observed with PMA treatment suggests that enhancing α secretase activity, and thereby promoting non-amyloidogenic processing of APP, could offer a therapeutic option for reducing Aβ accumulation in AD. In contrast, the increased Aβ secretion observed with BACE1 overexpression highlights both the importance and the challenges associated with targeting β secretase.
The results provide strong support for the potential of shifting APP processing toward the non-amyloidogenic pathway, where α secretase cleaves APP in a way that prevents the formation of Aβ peptides. This shift could have a profound impact on reducing amyloid plaque formation and the neurotoxic effects of Aβ accumulation. Given the evidence linking the accumulation of Aβ plaques to the progression of Alzheimer’s disease, promoting non-amyloidogenic processing by increasing α secretase activity could be a highly effective strategy for slowing or preventing disease progression. Previous research has demonstrated that activation of PKC, as with PMA treatment, can increase α secretase cleavage of APP, leading to a decrease in Aβ levels (Hung et al., 1993; Skovronsky et al., 2000). These findings align with our own, suggesting that PKC activation of α secretase could become a viable therapeutic strategy for reducing Aβ production without significantly affecting other cellular processes.
However, BACE1 is a more complex challenge, as its overexpression in our study resulted in a significant elevation in levels of Aβ. Inhibiting BACE1 as a therapeutic approach is important to consider due to its potential to decrease Aβ production and reduce amyloid plaque formation. Several small molecule inhibitors of BACE1 are currently under investigation in clinical trials but targeting BACE1 inhibition must be approached with caution. BACE1 is not only involved in APP processing but also plays essential roles in various physiological processes, such as synaptic plasticity, myelination, and neuronal survival (Cai et al., 2001). Inhibiting BACE1 too broadly may interfere with these critical functions, leading to unintended side effects. The challenge lies in developing BACE1 inhibitors that specifically target its role in APP processing without disrupting its other physiological functions. Achieving this balance is crucial to making BACE1 inhibition a viable therapeutic option for Alzheimer’s disease.
Another important consideration is the potential for a combination of therapies. Based on the competitive dynamics between α and β secretase pathways observed in our study, a dual therapeutic approach that combines enhancing α secretase activity while inhibiting β secretase could be a particularly effective strategy. By promoting non-amyloidogenic cleavage while also preventing amyloidogenic processing, this approach could potentially overcome some of the limitations associated with single-target therapies and provide a more synergistic reduction in Aβ levels.
The competitive nature of these pathways also suggests that APP processing is not a simple “on-off” switch between the two pathways but rather an equilibrium that can be influenced by various factors, including the availability of substrate, APP, and the activity of secretases. Due to this any therapeutic approach aimed at altering APP processing will need to account for this equilibrium and the potential for mechanisms that may regulate enzyme activity in response to changes in cellular conditions.
Limitations and Future Directions
One possible limitation of this study is the use of CHO695 cells, a non-neuronal cell line that expresses the 695 isoform of amyloid precursor protein (APP). While these cells provide a good model for studying APP processing and Aβ production, they may not fully depict the complexity of APP processing that occurs in neurons. Neurons possess a distinct set of cellular mechanisms and signaling pathways that can influence APP processing and Aβ secretion. Additionally, neurons express a different array of secretases, including isoforms of α and β secretase, which may vary in their activity and subcellular localization. Thus, the findings from this study may not completely reflect the physiological conditions found in the brain, particularly concerning neurodegenerative diseases like Alzheimer’s disease.
To address this limitation, future studies could replicate the effects observed in CHO695 cells using neuronal models, such as primary neurons or human induced pluripotent stem cell (iPSC)-derived neurons. These models would provide a more accurate representation of APP processing and Aβ secretion in a neuronal environment. Additionally, in vivo studies using animal models of AD would help confirm whether the findings from the cell culture experiments translate to more complex biological systems. These models could also account for the effects of other cellular interactions, such as microglia and astrocytes, which play an important role in the brain’s response to amyloid plaque accumulation and neurodegeneration. In vivo studies would also allow for the evaluation of therapeutic strategies, such as PMA treatment or BACE1 inhibition, within the context of the brain’s blood-brain barrier and neuroinflammatory responses.
Another limitation to consider is the specificity of PMA as a PKC activator. PMA is an activator of PKC, but it is known to activate multiple PKC isoforms, some of which can possibly have roles beyond the regulation of APP processing. For instance, PKC isoforms are involved in regulating processes such as cell proliferation, survival, and apoptosis. Therefore, while PMA effectively reduces Aβ secretion by promoting the α secretase pathway, its non-specific activation of various PKC isoforms raises the issue of possible side effects. These side effects could limit the therapeutic potential of PMA, especially if they lead to adverse outcomes. To enhance the potential of this approach, future studies should focus on developing and testing more selective PKC activators or analogs that specifically target the PKC isoforms involved in APP processing, while minimizing the activation of other pathways. Additionally, examining the downstream signaling pathways activated by PKC in the context of APP processing could provide more insights into how PKC selectively regulates APP cleavage without triggering unintended cellular responses.
Future studies could also investigate the combined effects of α secretase activation and β secretase inhibition. While our study demonstrates that PMA treatment and BACE1 overexpression have opposing effects on Aβ secretion, a more comprehensive approach may involve modulating both pathways simultaneously. Enhancing α secretase activity while inhibiting β secretase could synergistically reduce Aβ production, potentially leading to more effective reductions in the number and size of amyloid plaques. However, this combined approach needs to be explored further, as simultaneous modulation of both pathways can potentially lead to additional challenges, such as optimizing drug dosages or minimizing potential side effects. Additionally, investigating the role of other secretases, such as gamma-secretase, in regulating APP processing could further enhance our understanding of the proteolytic events that determine Aβ levels. Gamma secretase is the final enzyme in APP processing, and its activity may interact with both α and β secretase pathways. Understanding how these interactions affect the production of Aβ peptides could provide new therapeutic targets and lead to more refined strategies for treating Alzheimer’s disease.
Conclusion
In conclusion, our study provides compelling evidence of the opposing effects of PMA treatment and BACE1 transfection on APP processing and Aβ secretion. By demonstrating that PMA activates α secretase to reduce Aβ secretion and that BACE1 overexpression enhances Aβ production by promoting amyloidogenic APP processing, our findings highlight the competitive nature between α and β secretase pathways in regulating Aβ levels. These results contribute to the growing body of knowledge supporting the idea that modulating APP cleavage pathways could serve as a promising therapeutic strategy for Alzheimer’s disease.
Our findings suggest that PMA-induced PKC activation promotes a shift towards non-amyloidogenic APP processing, leading to a reduction in Aβ secretion. This mechanism of action could serve as a promising strategy for reducing amyloid plaque formation in Alzheimer’s disease. However, further studies in neuronal models and in vivo systems are necessary to confirm the therapeutic potential of PKC activation and evaluate its long-term effects. The study also underscores the challenges of targeting β secretase, as its inhibition needs to be precisely controlled to avoid disrupting its other essential functions. Future studies should aim to refine these approaches by developing more selective activators and inhibitors and validating their efficacy in more relevant models like neuronal models or in vivo systems.
The results of this study also provide important insights into the competitive nature between the α and β secretase pathways and their implications for Alzheimer’s disease pathology. While enhancing α secretase activity represents a promising strategy for reducing Aβ production and plaque formation, the challenges associated with β secretase inhibition highlight the need for selective approaches to modulate these pathways. Further studies will be necessary to refine these therapeutic strategies, address any potential side effects, and explore combination therapies that can more effectively balance the regulation of APP processing in Alzheimer’s disease.
Overall, a deeper understanding of the interactions between α, β, and γ-secretase pathways, as well as the combined effects of modulating these enzymes, could provide a more comprehensive approach to managing Aβ levels in Alzheimer’s disease. Ultimately, the development of therapies that precisely modulate APP processing to reduce Aβ production, while avoiding unintended side effects, hold great promise for improving treatment options for Alzheimer’s disease and other neurodegenerative disorders.
Note: Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College. Articles published within Eukaryon should not be cited in bibliographies. Material contained herein should be treated as personal communication and should be cited as such only with the consent of the author.