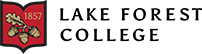Machine Learning Driven Detection and Pathology Validation of Parkinson’s Disease in Rodents: A Preliminary Model
*This author wrote this paper as a senior thesis under the direction of Dr. Eun Jung Hwang.
ABSTRACT
Animal models are crucial for neurological research as they allow researchers to elucidate complex neurological processes and develop treatments through controlled experimental trials. Most neurological disorders research using animal models involves two critical steps: evaluation of behavioral symptoms of the animal models and histological validation of pathologies. These two steps both require multiple human judges’ exquisite expertise in neurological diseases, for instance, behavioral shifts and cellular changes in anatomical structure. Such human-dependent methodologies are time-consuming, labor-intensive, and susceptible to human bias, undermining the robustness and reproducibility of animal research. In this project, I aimed to improve the efficiency, objectivity, and accuracy of animal model research by applying emerging artificial intelligence (AI) technologies to the analysis of behavioral and pathological data, focusing on the mouse models of Parkinson’s disease (PD). Using an AI tool DeepLabCut, I implemented an automatic body-pose extraction pipeline for the analysis of free-roaming behavior of PD mice. I also established a semi-automatic analysis pipeline for quantifying the dopaminergic neuron degeneration in the brain tissues of PD mice, using a machine-learning workflow QUINT. These AI-based pipelines significantly reduced human intervention in data analyses. Furthermore, using this semi-automatic analysis pipeline, I found a significant correlation between the extent of dopaminergic neuronal degeneration, a prime pathological feature in PD mice, and the severity of motor dysfunction computed from their behavioral data. The finding suggests that the progression of pathological processes may be reliably inferred from behavioral data. These results set a strong foundation for future endeavors to develop AI-based algorithms that are capable of computing behavioral symptoms and pathology scores directly from video recordings and brain images, a powerful tool for animal model research.
INTRODUTION
According to the 2024 World Health Organization report, one in three people will be affected by a neurological disorder at some point in their lifetime (WHO Media Team, 2024). This stark statistic highlights the pressing need to deepen our understanding of neurological disorders in order to improve disease prevention, treatment, and rehabilitation. Animal models have played pivotal roles in this endeavor as they allow in-depth investigation of brain pathogenesis associated with neurological disorders at multiple stages of disease progression, as well as underlying mechanisms at molecular and cellular levels. For example, MPTP-induced parkinsonian primate models have provided valuable insights into disease progression including the formation of Lewy bodies in dopaminergic (DA) neurons, synaptic alterations, mitochondrial dysfunction, and neuroinflammation/immune responses (Mat Taib & Mustapha, 2020). Animal models are also critical in diagnostic biomarkers development, therapeutic targets identification, and novel treatment testing. For instance, common PD treatment L-DOPA and recently developed deep brain stimulation were both developed and verified on primate models before clinical trials (Pereira & Aziz, 2006).
Most neurological disorders lack known biomarkers (e.g., antigens in blood) that readily and reliably provide information about the pathogenic processes or response to treatments. As a consequence, such information is mainly inferred from clinical behavior observations, a practice that prompts human judge bias and error. PD symptoms in animal models are scored based on observable symptoms like movement patterns and body postures. For example, open-field tests are used to assess general motor activities, based on simple features such as travel distance, and movement speed. The pole test is used to assess bradykinesia, and the cylinder test is used to measure sensory-motor coordination (Glajch et al., 2012). A reliable assessment requires a combination of tests, as well as a group of human judges with expertise who can interpret behavioral nuances, which requires both time and money (Asakawa et al., 2016). Besides, although human eyes can capture behavioral deviation, our visual sensitivity is limited. This limitation makes it difficult to study the early stages of diseases, a time usually associated with subtle symptoms, but the time to expect the highest intervention efficiency (Gaenslen & Berg, 2010). Furthermore, a prolonged duration of assessments could lead to fatigue in human judges and cause observer bias and low reproducibility.
These limitations also apply to the validation of disease models or treatment efficacy, which relies on behavioral assays and post-mortem brain histology. Such histological assessment of pathogenesis relies on human judges’ expert knowledge for differentiating anatomical structures and identifying disease-indicating features from brain tissue sections. For example, DA neuron quantification in assessing neurodegenerations in PD has been done manually under microscopes (Wakamatsu et al., 2008). Thus, the histological assay also suffers from the same issues as behavior assessment, posing general challenges in achieving the desirable level of efficiency and objectivity in animal model research.
To increase the sensitivity, effectiveness, and reproducibility of neurological disease research using animal models, we propose to use artificial intelligence (AI) technologies to outperform human judges in behavioral and histological analyses. In particular, we aim to apply AI algorithms to PD research using mice the dominant animal model used in the field.
1. Dopamine Neuron and Parkinson’s Disease
DA neurons are typically medium-sized neurons with elongated axons located in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNpc) of the midbrain (Dugan et al., 2011). These neurons synthesize DA, a monoamine neuromodulator that is involved in many brain functions. Although DA neurons only account for less than 1% of total neuron populations in animals (Chinta & Andersen, 2005), they play crucial roles in the reward system, motor function, working memory, motivation (Wise, 2004), emotion, learning (Redgrave & Gurney, 2006), and so on. They are also implicated in a diverse range of neurological and psychiatric disorders including PD, addiction, obsessive-compulsive disorder, and schizophrenia (Girault & Greengard, 2004). Beyond the central nervous system, DA receptors are also present in the kidneys, lungs, and blood vessels, contributing to pancreatic endocrine and insulin regulation (Volkow et al., 2008). These multifaceted involvements of DA highlight their significance in maintaining animal health.
The progressive loss of nigrostriatal innervation – DA neurons projecting their axons from the SNpc to the striatum - causes PD (Dickson, 2012). The majority of the existing research reports that the first PD motor symptom occurs only after ~50% of the DA neurons are lost, while higher thresholds have been also reported. Because DA neurons distinctively carry pigments called neuromelanin, which only forms when free DAs are present in the cytosol (Fedorow et al., 2005), DA-depleted brain display a loss of pigments in the SNpc and VTA region (Figure 1).
DA neurons are divided into three categories based on their anatomical locations: VTA - A10, SNpc - A9, and retrorubral field - A8 (German & Manaye, 1993). These anatomical divisions of neurons are also related to their projection targets and functional specificities, distinguishing DA neurons from other monoaminergic projections such as norepinephrine and serotonin (Avery & Krichmar, 2017). A9 DA neurons in the SNpc project to the dorsal striatum through the nigrostriatal pathway, which releases DA that modulates D1 and D2 receptors, each selectively expressed in striatal neurons in the “go” and “no-go” pathway, respectively (McGregor & Nelson, 2019). So A9 neurons are especially important for regulating voluntary motor control (Aubert et al., 1997) (Figure 2). In contrast, DA released from VTA and the retrorubral field play roles in regulating emotion, reward, addiction, and other cognitive functions. Besides anatomical structures, a better understanding of DA neurons’ genetic profiles can more comprehensively elucidate DA neurons’ diverse functionality. A recent study identified three subtypes of DA neurons based on their genetic profiles and their distinctive functional roles: Aldh1a1+, Calb1+, and Vglul+. A subset of the Aldh1a1+ subtype (Anxa1+) that projects to the dorsal striatum displays increased activity patterns that are temporally locked to the movement acceleration phase. The other subtypes projecting to the ventral striatum display deceleration-locked activity patterns in addition to reward responses. Therefore, diverse subtypes of DA neurons each with a distinct molecular profile, show different anatomical projection targets, and functional roles (Azcorra et al., 2022). In particular, a loss of Anxa1+ neurons might account for significant motor deficits in PD.
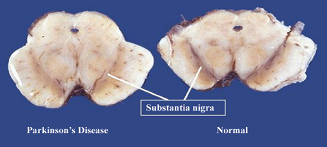
Figure 1: Substantia nigra pigment comparison between a PD patient and a healthy individual (Neveen A, 2019).
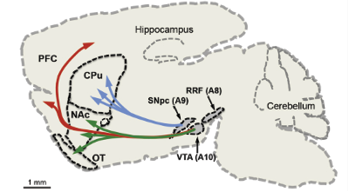
Figure 2: A8, A9, and A10 DA neuron clusters and their projections to caudate putamen (i.e., striatum), nucleus accumbens, olfactory tubercle, and prefrontal cortex (Luo & Huang, 2016)
2. Rodent Models of Parkinson’s Disease
2.1 Genetic PD model
Normal aggregation of alpha-synuclein is beneficial and essential for an organism, but excessive alpha-synuclein aggregation will lead to PD (Fields et al., 2019). Alpha-synuclein’s function is not clearly identified yet, but existing research suggests its role in maintaining the supply of synaptic vesicles in the presynaptic terminals and regulating the release of DA. Thus, the misfolding of alpha-synuclein will cause abnormal activities in these regards (Fields et al., 2019). There are multiple genetic mouse models that show alpha-synuclein aggregation. These models play a critical role in understanding the molecular processes underlying the aggregation of alpha-synuclein and their effects on behavioral functions.
The first gene discovered to underlie PD was the SNCA gene that encode alpha-synuclein protein, which is linked to familial PD (Polymeropoulos et al., 1997). SNCA is associated with an early-onset of PD. Mutations such as duplication, triplication, and point mutation of SNCA lead to the synthesis and misfolding of alpha-synuclein (Srinivasan et al., 2021). SNCA-mutated PD mice have provided evidence in understanding the altered DA release and uptake (Villar Piqué et al., 2015), olfactory dysfunction (Uemura et al., 2021), and rapid eye movement sleep disorders (Zhao et al., 2020) in PD. Besides SNCA mutation, alkaline phosphatase-related gene mutations, and ubiquitin-proteasome system-related gene mutations are also used in PD research (Pan et al., 2008). These genetic PD models have made significant contributions to the understanding of pathogenesis in PD (Siddiqui et al., 2016).
2.2 Neurotoxin PD Model
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) are commonly used chemical neurotoxins for inducing DA depletion and parkinsonian symptoms in animal models.
MPTP is a specific and reproducible neurotoxin that damages the nigrostriatal system. Its effectiveness has been shown in monkeys, mice, and rats (Tieu, 2011). MPTP can easily cross the blood-brain barrier and accumulate in DA neurons through DA transporters, so it can be injected subcutaneously in rodents. The accumulation of MPTP leads to ATP reduction and oxidative stress increment in neurons, thus causing cell death and neuroinflammation (Jackson-Lewis & Przedborski, 2007). While the MPTP method is robust and easy, the amount of MPTP administered needs to be delicately controlled because rodents are resistant to lower doses, but are susceptible to death with higher doses. Additionally, studies suggest that certain mouse strains can show recovery from acute motor deficits after MPTP injection within a few days (Sedelis et al., 2001). Therefore, MPTP is less ideal to use in long-term characterizations of the disease.
In contrast, 6-hydroxydopamine (6-OHDA) is an analog to DA and norepinephrine that depletes DA neurons by producing oxidative stress and reactive oxygen species within DA neurons (Tieu, 2011). It does not cross the brain-blood barrier, so it must be injected intracranially to affect neurons. Although the administration of 6-OHDA is more complicated, it allows specific targeting of DA depletion in the brain. For instance, small brain regions like the medial forebrain bundle can be targeted with 6-OHDA and leads to robust PD induction in mice. This cannot be done with genetic PD mice model or MPTP mice model. Additionally, injecting 6-OHDA into only one hemisphere of the brain allows animal models to mimic the asymmetrical nature of nigrostriatal dopaminergic innervation of PD progression in humans (Bové & Perier, 2012).
In the current study, we use 6-OHDA to induce DA neuronal degeneration and PD symptoms in mice. 6-OHDA enables us to target axons in the striatum that are projected from SNpc in only one hemisphere, so our mice model can emulate the asymmetric pathogenesis of human PD.
2.3 Behavior Analysis in PD Mice
Studying PD phenotypes in mice requires a combination of behavior observations and histological assessment. It is important to obtain valid animal behavior data because histological assessment can only be done after mice are sacrificed. I will discuss a few behavioral assays that are commonly used to assess the degree of motor deficits in PD mice compared to normal control mice below.
Open-field Test
The open-field test is the most widely used assay that examines the animal’s free locomotive activity. Across the literature and various mouse models,PD mice exhibit a decrease in locomotor activities in open-field tests (Taylor et al., 2010), except for A53T Synuclein Transgenics mice (Unger et al., 2006). During open-field tests, animals are placed in an open-field arena, where they freely roam for typically 10 minutes while being video recorded with an overhead camera. Human judges can analyze the animals’ general motor features such as total travel distance, average movement speed, and freezing behaviors from the recorded videos. Simple measures like the time the animal spends around the edge of the box can also be used to measure the level of anxiety associated with PD (Seibenhener & Wooten, 2015). Besides using video recording, infrared beam arrays are another way to track the animal’s locomotion. This method requires outfitting the open-field chamber with infrared beam sensors. As the animal moves and interrupts the photobeam, the infrared beam system can estimate the animal’s location and movement pattern (Klein et al., 2022). Photo-beam open-field recording requires less computation and less storage space with a more automated measurement of basic locomotor activities, but it does not allow the tracking of multiple animals if one intents to study animal interactions. While both methods can serve the same movement-tracking purpose, the video-recorded open-field test is more advantageous in providing comprehensive and detailed data for more complex body postures and movement patterns. Video-recorded open-field tests are also more suitable for recording multiple animals when assessing social interactions.
Grip Strength Test
The grip strength test is typically used to diagnose central nervous system disorder, It assesses the animal’s neuromuscular functions. PD mice tend to show a decrease in grip strength (Tillerson et al., 2002). In the test, the animal’s paws are placed on a wire grip, which they naturally hold on to. The human observers gently pull the rodent’s tail backwards and observe the gripping strength through the dynamometer connected with the wire grip. The maximal gripping strength before the rodent releases its paw from the grid will be recorded and used for neuromuscular function assessment.
Pole Test
The pole test is widely used for assessing bradykinesia (slowness of the movement) and unilateral brain lesions in PD mice. During the test, mice will be placed facing upward on a pole. The time that mice spent descending from the pole, and the number of rotations during descending motion are recorded. PD mice are shown to take longer to descend, exhibiting bradykinesia (Mitsumoto et al., 1998). Unilateral brain-lesioned mice additionally exhibit ipsilateral – the same direction as the lesioned brain hemisphere- turning when descending.
Drug-Induced Ipsilateral Rotation
Amphetamine is a stimulant drug that increases DA release. In humans, it is used to treat ADHD (Heal et al., 2013). In unilaterally lesioned PD mice (i.e., DA degeneration manifests in only one hemisphere), their body movements on the contralateral side of the lesioned hemisphere are impaired. The administration of amphetamine induces an excessive release of DA and leads to hyperactivity of the non-lesion sides of the brain. This hyperactivity of the non-lesion side of the brain will induce a dramatic ipsilateral turning behavior (Iancu et al., 2005). Conventionally, turning behaviors are counted by human judges’ visual inspections, posing a risk of subjective biases and errors.
Human judges play essential roles in PD diagnosis. Trained judges can use their expert knowledge to make valuable judgments about animal behavior patterns, thus detecting behavior changes and altering disease symptoms. However, even expert judges have limited visual sensitivity to subtle changes. Besides, manual measurements are also prone to biases and errors. To improve the efficacy of animal behavior analysis, scientists have developed artificial intelligence technology tools to detect and analyze animal behavior with higher sensitivity and objectivity, ideally to reduce the number of behavior assays and time spent for a single diagnosis. For instance, DeepLabCut is an automated video-tracking tool that comprehensively tracks animals’ body parts thus allowing researchers to analyze animal behavior patterns in depth.
3. Artificial Intelligence
3.1 Introduction of Artificial Intelligence
Artificial Intelligence (AI) has grown dramatically in the past twenty years and has transformed the way we live. The goal of AI is to simulate human intelligence to solve real-life problems. This rapid development portrays the current technological advancements and humanity’s growing desire to develop automated assistants that make life easier. According to John McCarthy, the development of an AI system can be either software, hardware, or both. AI’s key application elements are natural language processing, expert systems, robotics, intelligent agents, and computational intelligence (Collins et al., 2021) (Figure 3).
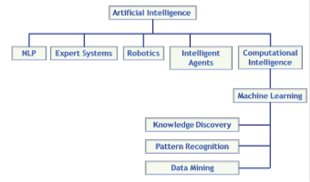
Figure 3: Key elements of the AI family tree. Source: Saudi Aramco
Natural language processing (NLP) is a machine-learning technology that enables computers to interpret, process, and comprehend human languages. Natural language processing (Nadkarni et al., 2011) has been used to develop chatbots like virtual nursing assistants. This technology takes tedious tasks off nurse’s shoulders so they can spend time in patient care. Nurses still interact with online clients but only clients request to speak with a real nurse. An expert system is a computer program that is refined and sufficient to provide diagnosis or treatment insights for disease. DXplain developed by Massachusetts General Hospital/Harvard Medical School Laboratory of Computer Science is based on over 2600 diseases and 5700 clinical findings. It has become a staple use in hospitals and medical schools for clinical education (Barnett, 1987). However, despite AI’s rapid development, human clinical AI technology is not yet independent, as it must be used in combination with human doctors. In contrast, robotic-assisted stereotaxic surgery has been used for microinjection and even craniotomy in animal research (Ball et al., 2021). This robotics substantially automates the surgery process compared to the traditional manual approach. Yet, human supervision is still required and necessary. Lastly, an intelligent agent is a program that can make decisions based on the environment, user input, and experience (Engelbrecht, 2023). IBM for Oncology has been used in real life. This program can combine a patient’s file, clinical expertise, external research, and data to identify and rank the treatment plans and options.
Various AI elements have become tools in healthcare. From backstage paperwork to face-to-face patient care, the collaboration between AI and human efforts improves the efficiency of different processes. Most AI applications are built on large datasets, thus requiring excellent efficient data processing and analyzing techniques, like machine learning.
3.2 Machine Learning Techniques
Machine learning (ML) is the most popular technique in AI, where statistical models make inferences based on datasets going so far as to mimic human decision-making in very specific instances. The goal of machine learning is to interpret and learn from data. Without explicit coding, ML will play the knowledge-discovery and data-mining role to derive values from data. ML can automatically improve itself over time with more training and data (Nasteski, 2017). With the rising demands for data processing, machine learning has been applied widely in our daily lives, such as image recognition and speech recognition from personalized recommendations on platforms such as Google, Spotify, and Netflix.
3.2.1 Supervised Machine Learning
Supervised ML learns patterns from data based on input-output pairs. “Supervised” is derived from the fact that output for training data is given. To train a supervised ML algorithm, input data are split into training and testing datasets. The training dataset will include labels that indicate classifications, such as male vs. female, big vs. small, or 1 vs. 0. This labeled dataset is used to train the algorithms. The algorithm will pick up data pattern differences relative to their labeling. After sufficient training and evaluation, the algorithm is used to predict the test dataset. Adjustments might be made if prediction performance is not ideal (Figure 4) (Nasteski, 2017).
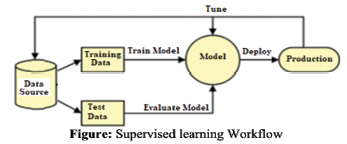
Figure 4: Supervised Learning Workflow. Source: https://pub.aimind.so/supervised-machine-learning-algorithms-and-techniques-explained-in-depth-2a904fe77ee3
3.2.2 Supervised Machine Learning: Linear Regression
Ordinary least squares regression (OLS) is the simplest regression method. Its goal is to capture the best-fit linear relationship (coefficients and intercept) between dependent and independent variables. The linear model calculates the coefficients (a, b, c, d…) of each independent variable (x1, x2, x3, x4…). The weighted function (f(x) = ax1 + bx2 + cx3 + dx4…) represents the optimal linear relationship. To evaluate its performance, the sum of squared error is used. The difference between the predicted data point and the actual data point is known as the residual or error. Taking the sum of the squared residual/error of different weighted functions select the best linear unbiased estimator (BLUE) of a linear relationship. Because OLS regression relies on minimizing the residual sum of squares, the performance of these models are typically evaluated by the mean squared error – an equivalent matric. The lower the mean squared error, the better the regression model (Figure 5). Even though the vast majority of relationships in the natural world are far more complex than linear relationships, nonlinear relationships have linear components. Because ordinary least squares regression has an optimal, closed-form solution, it provides better guarantees than any other ML model of discovering a linear component (Hastie et al., 2017).
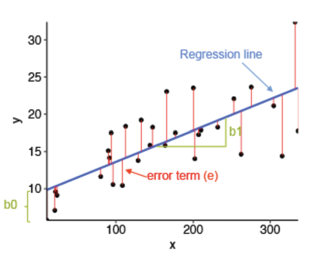
Figure 5: Linear Regression and its residual error. Source: https://rpubs.com/cuborican/regression
3.2.3 Supervised Machine Learning: Feedforward Neural Network
A feedforward neural network is a more complicated form of supervised machine learning. It is used when many independent variables are used to predict dependent variables. Different from linear regression, a neural network is used for nonlinear relationship prediction. In the brain, each neuron has its role in information processing, and they propagate signals back and forth. In a neural network, we articulate neurons of individual roles. The leftmost layer is the input layer that takes input features, and its size is typically the size of input data. After this layer, the hidden layer(s) will transform the input layer’s data using the weights of the hidden layer’s neurons Often, the more complicated a dataset is, the more hidden layers and neurons it will require. This computation propagates till data passes from the input layer to the last hidden layer, where a non-linear activation function will map current data into a known range, so the output layer can receive the information and transform it into output values. With competitive training, the weights of each hidden layer’s neuron
can be tuned to obtain a “better” model (Figure 6) (Hastie et al., 2017).
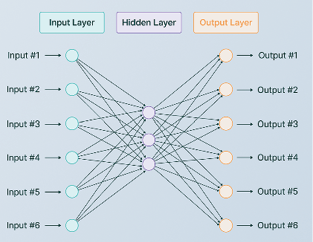
Figure 6: Neural Network Model Architecture. Source: https://www.v7labs.com/blog/neural-network-architectures-guide
Continuous training of neural networks has the potential to recognize and model complex relationships from the dataset. However, neural networks are highly prone to overfitting, thus it requires a large data set, if not thousands, or even millions of data points to minimize the risk of overfitting. Besides, there are some other disadvantages when applying supervised neural networks: 1) neural networks take time and money, computing power, and the tuning of the algorithm; 2) neural networks’ algorithms are mysterious, it is yet to know the mechanism of neural network’s decision-making process. Although neural networks might show incredible results, their unknown nature should be taken cautiously especially if it is used in real life.
4. Artificial Intelligence in Biomedical Research
4.1 AI Application in Human PD Diagnosis
Recent research has leveraged machine learning (ML) techniques to detect PD symptoms in human individuals, aiming to enable earlier diagnosis and monitoring of disease progression. These ML algorithms have demonstrated promising results in analyzing various motor PD symptoms for diagnosis, particularly using data from wearable sensors that capture relevant movement patterns from different body parts. Keijsers et al. used six triaxial accelerometers ( devices that provide simultaneous acceleration measurements in three orthogonal directions) that can be attached to different human PD patients’ body parts to collect their motor movement data (both upper arms, both upper legs, the wrist of the most affected body side, and the trunk) in a simulated house for 3 hours while PD patients conducting daily activities. With the comprehensive data collected from each sensor, Keijsers et al. characterized the mean segment velocity, mean segment velocity when moving, percentage of moving, and the percentage of dominant frequency per body part. They developed a neural network with two hidden layers, inputting all body parts’ characterized data. As a result, this neural network achieved a 97% of sensitivity and specificity in distinguishing the on and off state of the disease (Keijsers et al., 2006). This study reveals how integrated analysis of motor movement patterns can facilitate the detection of PD with the help of ML. Interestingly, another group of researchers investigated whether using less wearable devices to characterize one specific PD symptom can be used in PD detection. Hssayeni et al. estimated tremors using two sensor trackers (one on the wrist and one on the ankle) and correlated it with PD diagnosis under a more natural environment. Data was collected when participants conducted daily activities like walking, chopping vegetables, and getting groceries. By using a deep learning model, they showed the highest up-to-date correlation between PD diagnosis and tremor (Hssayeni et al., 2019). These studies show the potential of ML in improving the efficiency and sensitivity in diagnosing PD based on the patient’s motor behavior.
Building upon these findings, Aich et al. (2020) developed a method for identifying PD patients without wearable sensors. They applied 3D motion capture techniques to analyze the gait of participants without wearing any sensors. The 3D motion analysis system can capture participants’ motor activities based on participants’ video recordings in the natural environment. After obtaining data using 3D motion analysis, Aich et al. (2020) applied feature selection techniques to determine what motor features are significantly related to the disease and developed a ML classification algorithm based on these identified motor features. Their model was able to achieve an accuracy of over 98.56% in identifying PD patients (Aich et al., 2020). This study demonstrates the potential of ML in automating PD diagnosis through non-invasive techniques in the natural environment.
4.2 AI Application in Animal Research
4.2.1 Behavioral Data Analysis: DeepLabCut
AI technologies have been introduced and actively utilized in animal research. For instance, DeepLabCut (DLC) is an open-source software package used for tracking animal body parts and estimating poses without markers using deep learning. This program utilizes a combination of feature detectors (ResNets, readout layers, MobileNetV2s, etc) to obtain a state-of-the-art algorithm performing pose estimation (Nath et al., 2019) at an inexpensive cost. DLC processes video data from camera recording and performs body parts labeling and tracking based on a small set of user input. As a result, it exports files with x and y coordinates for each body part and timestamps. DLC can be applied to a wide range of organisms. DLC was used in Hayakawa et al’s research in studying crickets’ circadian rhythms. DLC successfully recognized and labeled crickets’ six body parts with high confidence during daily activities, thus providing evidence showing crickets’ diurnal rhythms (Hayakawa et al, 2024). Studying animal behaviors in the wild can interrupt animal’s natural behavior and cause danger to the researcher. DLC can help with this issue. Wiltshire et al utilized DLC to label chimpanzees and bonobos’ free-living behaviors, offering valuable kinematic data in studying wild primates (Wiltshire et al., 2023). DLC utilizes an elegant graphic user interface (GUI) that is easy to use, all the training can be done by mouse-clicking, and no coding knowledge is required to use DLC (Figure 7).
Figure 7: DeepLabCut
4.2.2 Histology Data Analysis: QUINT
Besides behavioral observation, the analysis of the animal brain tissue is also an important component in neurological disease research because they can provide critical pathological evidence of the disease. The common approach to studying the pathological processes of neurodegenerative disorders is to visualize specific molecule aggregates or loss by applying immunohistochemistry or other staining techniques on sectioned brain slices. The processed brain tissues are observed under a microscope to detect molecular/structural changes. Such brain tissue analyses involve two essential tasks: 1) anatomical identification of different structures and 2) protein/target molecule quantification. These two steps rely on domain-expert decisions and a large amount of manual operation, which makes the task difficult and time-consuming. Addressing these challenges in histology, a recently developed QUINT workflow provides semi-automatic feature quantification and analysis tools. Its efficacy has been demonstrated in many research. For instance, QUINT quantified the co-labeling SNCA and alpha-synuclein and revealed the revealed alpha-synuclein’s accumulation specificity in selected neuron types. QUINT is also capable of labeling multiple targets of interest (Geertsma et al., 2024). In Gurdon et al.’s research on Alzheimer’s disease, QUINT was used to label different neurons, microglial, reactive astrocytes, all nuclei, and beta-amyloid 1-42 pathology. These targets of interest are quantified in Alzheimer’s disease mouse models, revealing that the variation of age at onset and symptoms are related to cell compositions (Gurdon et al. 2023). QUINT workflow is a powerful tool with high efficiency in tissue registering and quantifying the pathogenic features in animal research.
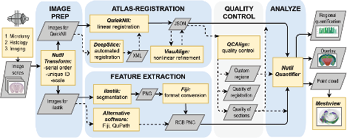
Figure 8: QUINT workflow
GOAL
I aim to build an AI-based analysis pipeline that semi-automatically processes behavior and histology data from PD mouse models, to improve efficiency, objectivity, and precision in the research of neurological disorders using animal model.
SPECIFIC AIMS
Establish a surgical protocol to generate PD mice.
Develop an automatic pipeline to analyze PD mouse movement data using DLC.
Establish an immunohistochemistry (IHC) protocol for histological assay of PD pathology.
Develop a semi-automatic pipeline to analyze IHC data using QUINT.
Develop an ML algorithm to diagnose PD from movement data.
Note: Eukaryon is published by students at Lake Forest College, who are solely responsible for its content. The views expressed in Eukaryon do not necessarily reflect those of the College. Articles published within Eukaryon should not be cited in bibliographies. Material contained herein should be treated as personal communication and should be cited as such only with the consent of the author.